Changing The Ph Can Cause A Protein To:'
Muz Play
Mar 25, 2025 · 6 min read
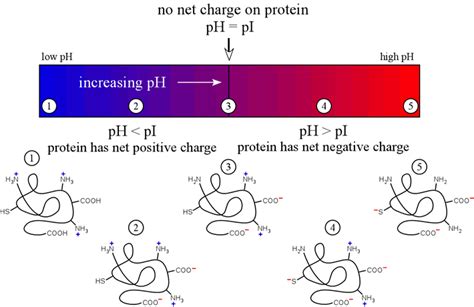
Table of Contents
Changing the pH Can Cause a Protein to: Denature, Misfold, and More
Proteins are the workhorses of life, carrying out a vast array of functions within cells and organisms. Their intricate three-dimensional structures are crucial to their activity, and even subtle changes to their environment can have profound consequences. One such environmental factor is pH, the measure of acidity or alkalinity. Changing the pH can cause a protein to denature, misfold, aggregate, or even undergo other structural alterations, significantly impacting its function and potentially leading to disease. Understanding how pH affects protein structure and function is critical in various fields, including medicine, biotechnology, and food science.
The Importance of Protein Structure and Function
Proteins are chains of amino acids linked together by peptide bonds. The sequence of these amino acids, the primary structure, dictates how the protein folds into its unique three-dimensional shape. This three-dimensional structure is crucial because it determines the protein's function. Proteins achieve their functionalities through intricate interactions with other molecules, including substrates, enzymes, and receptors. These interactions are highly specific and rely on the precise arrangement of amino acid side chains, which are influenced by the protein's three-dimensional structure.
Proteins adopt several levels of structural organization:
- Primary Structure: The linear sequence of amino acids.
- Secondary Structure: Local folding patterns such as alpha-helices and beta-sheets, stabilized by hydrogen bonds.
- Tertiary Structure: The overall three-dimensional arrangement of a polypeptide chain, stabilized by various interactions including hydrophobic interactions, disulfide bridges, and ionic bonds.
- Quaternary Structure: The arrangement of multiple polypeptide chains in a protein complex.
How pH Changes Affect Protein Structure
The pH of the surrounding environment significantly influences the charge distribution on the amino acid side chains of a protein. Many amino acid side chains possess ionizable groups, meaning they can gain or lose a proton (H+) depending on the pH. These ionizable groups include carboxyl groups (-COOH), amino groups (-NH2), and imidazole groups (in histidine).
-
Acidic pH (low pH): At low pH values, there is a high concentration of protons (H+). This leads to the protonation of many ionizable groups on the amino acid side chains. For example, carboxyl groups become protonated (-COOH), acquiring a neutral charge. This shift in charge can disrupt the electrostatic interactions that stabilize the protein's tertiary and quaternary structures.
-
Basic pH (high pH): At high pH values, there is a low concentration of protons (H+). This leads to the deprotonation of many ionizable groups. For instance, amino groups lose their protons (-NH2 becomes -NH-), acquiring a negative charge. Again, this change in charge distribution can disrupt the electrostatic interactions that maintain the protein's structure.
The Process of Protein Denaturation
When the pH deviates significantly from a protein's optimal range, it can lead to denaturation. Denaturation is the process where a protein loses its native three-dimensional structure and unfolds. This unfolding disrupts the protein's function because the specific arrangement of amino acid side chains required for interactions with other molecules is lost.
Several factors contribute to denaturation at extreme pH values:
- Disruption of electrostatic interactions: Changes in the charge distribution on amino acid side chains alter the electrostatic interactions that stabilize the protein's structure. This can lead to the unfolding of the protein.
- Disruption of hydrogen bonds: Changes in pH can also disrupt hydrogen bonds, which are important for maintaining the secondary structure of proteins.
- Exposure of hydrophobic residues: In the native state, hydrophobic amino acid residues are usually buried within the protein's core. Denaturation exposes these residues to the aqueous environment, leading to aggregation.
Consequences of Protein Denaturation and pH-Induced Changes
The consequences of protein denaturation due to pH changes can be severe, impacting a wide range of biological processes:
-
Loss of enzymatic activity: Enzymes are proteins that catalyze biochemical reactions. Denaturation renders enzymes inactive because their active sites, the regions where substrates bind, are distorted. This can disrupt metabolic pathways and cellular functions.
-
Loss of structural integrity: Proteins provide structural support to cells and tissues. Denaturation can weaken or destroy this structural support, affecting the integrity of cellular components and tissues.
-
Protein aggregation: Denatured proteins can aggregate, forming insoluble clumps. These aggregates can interfere with cellular processes and contribute to the development of various diseases, including Alzheimer's disease and Parkinson's disease. These aggregates can disrupt cellular function and lead to cellular stress and death.
-
Immune response: Denatured proteins can be recognized as foreign substances by the immune system, triggering an immune response. This can lead to inflammation and autoimmune diseases.
-
Changes in protein solubility: Changes in pH can alter a protein's solubility. Some proteins become less soluble at extreme pH values, potentially leading to precipitation or aggregation.
-
Impact on protein-protein interactions: Protein function often relies on interactions with other proteins. pH changes can disrupt these interactions, affecting signaling pathways, cellular communication, and other crucial biological processes.
Examples of pH-Dependent Protein Function
Many proteins exhibit significant pH dependence in their function. Here are some examples:
-
Enzymes in the digestive system: Digestive enzymes like pepsin (active in the stomach's acidic environment) and trypsin (active in the alkaline environment of the small intestine) have optimal pH ranges for their activity. Changes in the pH of these environments can significantly impact their catalytic efficiency.
-
Hemoglobin: Hemoglobin, the oxygen-carrying protein in red blood cells, displays pH-dependent oxygen binding. The Bohr effect describes the phenomenon where a decrease in pH (increased acidity) reduces hemoglobin's affinity for oxygen, facilitating oxygen release in tissues.
-
Ion channels: Many ion channels in cell membranes are sensitive to pH changes. Altering the pH can affect their opening and closing, modifying the flow of ions across the membrane and impacting cellular excitability and signaling.
Buffer Systems and pH Homeostasis
Living organisms have evolved sophisticated mechanisms to maintain a stable internal pH, a process called pH homeostasis. This is crucial for preventing damage to proteins and other cellular components. Buffer systems, consisting of weak acids and their conjugate bases, play a vital role in resisting pH changes. These systems absorb excess H+ or OH- ions, minimizing fluctuations in pH and ensuring that proteins remain in their functional conformations.
pH Manipulation in Biotechnology and Medicine
The understanding of how pH affects proteins has led to various applications in biotechnology and medicine:
-
Protein purification: pH adjustments are frequently used during protein purification processes to alter the solubility or charge of proteins, facilitating their separation from other molecules.
-
Enzyme engineering: Researchers can modify the amino acid sequence of enzymes to alter their optimal pH range, making them more suitable for specific applications.
-
Drug delivery: The pH of the environment can be used to control the release of drugs. For example, pH-sensitive polymers can be used to encapsulate drugs, releasing them only in specific environments with a particular pH.
-
Treatment of diseases: In some cases, targeting the pH of specific cellular compartments or tissues can be used therapeutically to disrupt the function of disease-causing proteins or pathogens.
Conclusion
pH is a critical environmental factor influencing protein structure and function. Significant deviations from a protein's optimal pH range can lead to denaturation, misfolding, aggregation, and a loss of function. Understanding the intricate interplay between pH and protein structure is essential for various scientific disciplines. Further research into these interactions will continue to yield valuable insights with implications for medicine, biotechnology, and our fundamental understanding of biological processes. The impact of pH on proteins extends far beyond the lab, affecting the stability of food, the efficacy of pharmaceuticals, and even the pathogenesis of disease. Therefore, appreciating the pivotal role of pH in the world of proteins remains crucial for future advancements.
Latest Posts
Latest Posts
-
When Elements Combine To Form Compounds
Mar 25, 2025
-
Describe The Sampling Distribution Of P Hat
Mar 25, 2025
-
How To Determine State Of Matter In Chemical Equation
Mar 25, 2025
-
Resistors In Series And Parallel Calculator
Mar 25, 2025
-
Where Are Breathing Control Centers Located
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Changing The Ph Can Cause A Protein To:' . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
