Chemical Reactions Can Be Classified Based On Changes In Chemical
Muz Play
Mar 21, 2025 · 8 min read
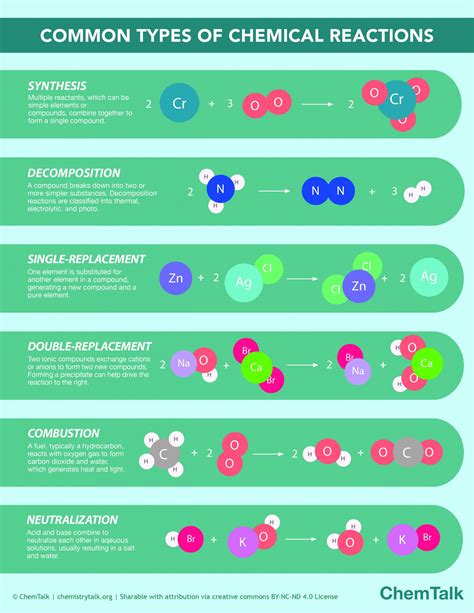
Table of Contents
Chemical Reactions: A Classification Based on Chemical Changes
Chemical reactions are the fundamental processes that govern the transformation of matter. Understanding how to classify these reactions is crucial in chemistry, allowing for predictions of reaction outcomes, the design of synthetic pathways, and a deeper understanding of the underlying chemical principles. This article delves into the various ways we can categorize chemical reactions based on the changes in chemical composition and properties that occur during the process. We'll explore common reaction types, providing examples and highlighting the key changes that define each category.
Major Classification Schemes
Chemical reactions can be classified in numerous ways, each offering a unique perspective on the transformation process. Some of the most common schemes include:
-
Based on the type of change: This categorization focuses on whether the reaction involves the formation of new substances (synthesis) or the breaking down of existing substances (decomposition). Further sub-classifications exist within these categories.
-
Based on the transfer of electrons: This approach examines the movement of electrons between reactants, leading to distinctions between oxidation-reduction (redox) reactions and other reaction types.
-
Based on the nature of the reaction: This encompasses a broad range of classifications, including acid-base reactions, precipitation reactions, and complexation reactions, each governed by distinct chemical principles.
We will explore these classification schemes in detail in the following sections.
1. Classification Based on the Type of Change: Synthesis and Decomposition Reactions
This fundamental classification distinguishes between reactions that build up new compounds (synthesis) and those that break down existing compounds (decomposition).
1.1 Synthesis Reactions (Combination Reactions)
Synthesis reactions involve the combination of two or more reactants to form a single, more complex product. The general form of a synthesis reaction is:
A + B → AB
Where A and B are reactants, and AB is the product. These reactions often involve the formation of new chemical bonds.
Examples of Synthesis Reactions:
-
Formation of water: 2H₂ + O₂ → 2H₂O Two simpler molecules of hydrogen and oxygen combine to form water. This is a highly exothermic reaction, releasing a significant amount of energy.
-
Formation of magnesium oxide: 2Mg + O₂ → 2MgO Magnesium reacts with oxygen to produce magnesium oxide, a common synthesis reaction involving a metal and a nonmetal.
-
Formation of ammonia: N₂ + 3H₂ → 2NH₃ Nitrogen gas and hydrogen gas react under specific conditions (high temperature and pressure) to yield ammonia, a crucial component of fertilizers.
Key Chemical Changes in Synthesis Reactions:
- Formation of new chemical bonds: New bonds are created between the atoms of the reactants, forming a more complex product.
- Increase in molecular complexity: The product is generally more complex than the reactants, having a higher molecular weight.
- Change in physical properties: The physical properties of the product (melting point, boiling point, solubility) are often significantly different from those of the reactants.
1.2 Decomposition Reactions
Decomposition reactions are the opposite of synthesis reactions. They involve the breakdown of a single, more complex reactant into two or more simpler products. The general form is:
AB → A + B
Where AB is the reactant, and A and B are the products. These reactions often require energy input, such as heat, light, or electricity.
Examples of Decomposition Reactions:
-
Electrolysis of water: 2H₂O → 2H₂ + O₂ Passing an electric current through water breaks it down into hydrogen and oxygen gas.
-
Thermal decomposition of calcium carbonate: CaCO₃ → CaO + CO₂ Heating calcium carbonate (limestone) decomposes it into calcium oxide (quicklime) and carbon dioxide.
-
Decomposition of hydrogen peroxide: 2H₂O₂ → 2H₂O + O₂ Hydrogen peroxide decomposes into water and oxygen, often catalyzed by enzymes or heat.
Key Chemical Changes in Decomposition Reactions:
- Breaking of chemical bonds: Existing bonds in the reactant molecule are broken, resulting in simpler products.
- Decrease in molecular complexity: The products are generally simpler than the reactant, having lower molecular weights.
- Change in physical properties: Similar to synthesis reactions, the physical properties of the products are different from the reactant.
2. Classification Based on Electron Transfer: Redox Reactions
Redox reactions, short for reduction-oxidation reactions, are characterized by the transfer of electrons between reactants. One reactant undergoes oxidation (loss of electrons), while another undergoes reduction (gain of electrons). These reactions are fundamental in many chemical and biological processes.
Oxidation: Loss of electrons, often accompanied by an increase in oxidation state.
Reduction: Gain of electrons, often accompanied by a decrease in oxidation state.
Examples of Redox Reactions:
-
Rusting of iron: 4Fe + 3O₂ → 2Fe₂O₃ Iron loses electrons (oxidation) to oxygen, which gains electrons (reduction), forming iron oxide (rust).
-
Combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O Methane is oxidized, losing electrons to oxygen, which is reduced, forming carbon dioxide and water.
-
Reaction of zinc with hydrochloric acid: Zn + 2HCl → ZnCl₂ + H₂ Zinc loses electrons (oxidation) to hydrogen ions, which gain electrons (reduction), producing zinc chloride and hydrogen gas.
Key Chemical Changes in Redox Reactions:
- Change in oxidation states: The oxidation states of the atoms involved change during the reaction.
- Electron transfer: Electrons are transferred from one reactant (reducing agent) to another (oxidizing agent).
- Changes in chemical properties: The chemical properties of the reactants change significantly due to the electron transfer.
3. Classification Based on the Nature of the Reaction
This broad category encompasses several specific reaction types, each defined by unique chemical mechanisms and characteristics.
3.1 Acid-Base Reactions (Neutralization Reactions)
Acid-base reactions involve the transfer of protons (H⁺ ions) from an acid to a base. The reaction typically produces salt and water.
Examples of Acid-Base Reactions:
-
Reaction of hydrochloric acid with sodium hydroxide: HCl + NaOH → NaCl + H₂O Hydrochloric acid (strong acid) reacts with sodium hydroxide (strong base) to produce sodium chloride (salt) and water.
-
Reaction of acetic acid with ammonia: CH₃COOH + NH₃ → CH₃COONH₄ Acetic acid (weak acid) reacts with ammonia (weak base) to form ammonium acetate.
Key Chemical Changes in Acid-Base Reactions:
- Proton transfer: Protons are transferred from the acid to the base.
- Formation of salt and water: The products are usually a salt (ionic compound) and water.
- Change in pH: The pH of the solution changes significantly depending on the strength of the acid and base involved.
3.2 Precipitation Reactions
Precipitation reactions involve the formation of a solid precipitate when two aqueous solutions are mixed. This precipitate is an insoluble ionic compound.
Examples of Precipitation Reactions:
-
Reaction of silver nitrate with sodium chloride: AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq) Mixing silver nitrate and sodium chloride solutions produces a white precipitate of silver chloride.
-
Reaction of barium chloride with sulfuric acid: BaCl₂(aq) + H₂SO₄(aq) → BaSO₄(s) + 2HCl(aq) Barium sulfate, an insoluble white precipitate, is formed.
Key Chemical Changes in Precipitation Reactions:
- Formation of a solid precipitate: An insoluble ionic compound forms and separates from the solution.
- Change in solution clarity: The solution becomes cloudy or opaque due to the precipitate formation.
- Ionic bonding: The precipitate is formed due to the strong electrostatic attraction between oppositely charged ions.
3.3 Single Displacement Reactions
Single displacement reactions (also known as single replacement reactions) involve the replacement of an element in a compound by another element. This usually occurs when a more reactive element displaces a less reactive element from a compound.
Examples of Single Displacement Reactions:
-
Reaction of zinc with copper(II) sulfate: Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s) Zinc, being more reactive than copper, displaces copper from copper(II) sulfate.
-
Reaction of chlorine with sodium bromide: Cl₂(g) + 2NaBr(aq) → 2NaCl(aq) + Br₂(l) Chlorine displaces bromine from sodium bromide.
Key Chemical Changes in Single Displacement Reactions:
- Replacement of an element: One element replaces another element in a compound.
- Changes in oxidation states: The displaced element often undergoes oxidation, while the replacing element undergoes reduction.
- Change in chemical properties: The properties of the resulting compound are different from the original compound.
3.4 Double Displacement Reactions (Metathesis Reactions)
Double displacement reactions (also known as metathesis reactions) involve the exchange of ions between two compounds. These reactions often occur in aqueous solutions and can lead to the formation of a precipitate, gas, or weak electrolyte.
Examples of Double Displacement Reactions:
-
Reaction of silver nitrate with potassium chloride: AgNO₃(aq) + KCl(aq) → AgCl(s) + KNO₃(aq) Silver chloride, an insoluble precipitate, is formed.
-
Reaction of sodium carbonate with hydrochloric acid: Na₂CO₃(aq) + 2HCl(aq) → 2NaCl(aq) + H₂O(l) + CO₂(g) Carbon dioxide gas is released.
Key Chemical Changes in Double Displacement Reactions:
- Exchange of ions: Ions are exchanged between two compounds.
- Formation of a precipitate, gas, or weak electrolyte: One of the products is usually insoluble, gaseous, or a weak electrolyte.
- Changes in solution properties: The solution properties, such as conductivity, may change significantly.
3.5 Combustion Reactions
Combustion reactions are exothermic redox reactions involving the rapid reaction of a substance with an oxidant, usually oxygen, to produce heat and light.
Examples of Combustion Reactions:
-
Combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O Methane reacts with oxygen to produce carbon dioxide, water, and heat.
-
Combustion of propane: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O Propane burns in oxygen to form carbon dioxide, water, and heat.
Key Chemical Changes in Combustion Reactions:
- Rapid oxidation: The fuel is rapidly oxidized by oxygen.
- Release of heat and light: A large amount of heat and light energy is released.
- Formation of oxides: The products are usually oxides of the elements present in the fuel.
Conclusion
Classifying chemical reactions helps us understand and predict the behavior of matter. By analyzing the changes in chemical composition and properties, we can group reactions into meaningful categories. This classification scheme, although diverse, provides a framework for analyzing complex chemical transformations and offers valuable insights into the underlying principles governing these processes. Further research and study into specific reaction mechanisms can reveal additional layers of complexity and nuance within each category. This article has provided a solid foundation for understanding the diverse and fascinating world of chemical reactions.
Latest Posts
Latest Posts
-
Compare A Human And Chimpanzee Skeleton Worksheet Answers
Mar 22, 2025
-
3 X 2 Factorial Design Example
Mar 22, 2025
-
What Type Of Solid Is Diamond
Mar 22, 2025
-
Do Electrons Have A Smaller Mass Than Protons And Neutrons
Mar 22, 2025
-
What Is The Building Block Of A Nucleic Acid Called
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Chemical Reactions Can Be Classified Based On Changes In Chemical . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
