Classify The Phase Changes As Endothermic Or Exothermic
Muz Play
Mar 16, 2025 · 7 min read
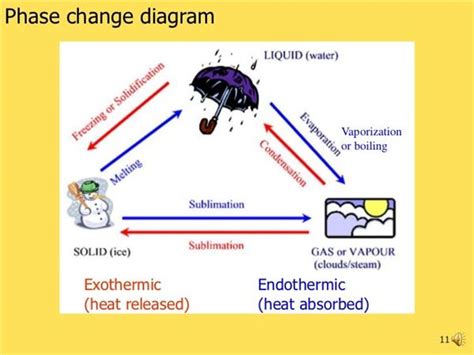
Table of Contents
Classify the Phase Changes as Endothermic or Exothermic
Phase changes, also known as phase transitions, are physical processes that involve a change in the state of matter of a substance. These changes are accompanied by the absorption or release of energy, classifying them as either endothermic or exothermic processes. Understanding the nature of these changes is crucial in various scientific fields, including chemistry, physics, and meteorology. This comprehensive guide will delve into the classification of phase changes, explaining the underlying principles and providing real-world examples.
Understanding Endothermic and Exothermic Processes
Before classifying phase changes, it's essential to understand the fundamental difference between endothermic and exothermic processes. These terms describe the energy exchange between a system and its surroundings.
-
Endothermic Processes: These processes absorb energy from their surroundings. The system's energy increases, resulting in a decrease in the temperature of the surroundings. Think of it like a sponge soaking up water – the sponge (system) gains energy, and the water (surroundings) loses energy.
-
Exothermic Processes: These processes release energy to their surroundings. The system's energy decreases, resulting in an increase in the temperature of the surroundings. Imagine a burning candle – the candle (system) releases energy as heat and light, warming the surrounding air.
Classifying Phase Changes
Now, let's classify the common phase changes: melting, freezing, vaporization (boiling and evaporation), condensation, sublimation, and deposition.
1. Melting (Solid to Liquid) – Endothermic
Melting involves the transition of a substance from a solid state to a liquid state. This process requires energy input to overcome the intermolecular forces holding the solid's particles together in a fixed structure. The energy absorbed weakens these bonds, allowing the particles to move more freely, characteristic of a liquid.
Example: Ice melting into water. The ice absorbs heat from the surroundings (e.g., room temperature air), causing its temperature to increase until it reaches its melting point (0°C at standard pressure). Further heat absorption breaks the hydrogen bonds in the ice crystal lattice, resulting in the formation of liquid water.
2. Freezing (Liquid to Solid) – Exothermic
Freezing is the opposite of melting. It involves the transition of a substance from a liquid state to a solid state. During freezing, the particles in the liquid lose energy, causing them to slow down and their kinetic energy to decrease. This allows the intermolecular forces to draw the particles closer together, forming a rigid structure characteristic of a solid. The energy released during freezing is the energy that was previously absorbed during melting.
Example: Water freezing into ice. As water cools, its particles lose kinetic energy. Once it reaches its freezing point (0°C at standard pressure), the energy released during the phase transition forms a stable crystalline ice structure. This released energy warms the surrounding environment slightly.
3. Vaporization (Liquid to Gas) – Endothermic
Vaporization encompasses two related processes: boiling and evaporation. Both involve the transition of a substance from a liquid state to a gaseous state. This requires significant energy input to overcome the strong intermolecular forces holding the liquid molecules together. The absorbed energy increases the kinetic energy of the molecules, allowing them to escape the liquid's surface and enter the gaseous phase.
-
Boiling: Boiling occurs at a specific temperature called the boiling point, where the vapor pressure of the liquid equals the external pressure. Bubbles of vapor form within the liquid and rise to the surface.
-
Evaporation: Evaporation occurs at temperatures below the boiling point. It is a surface phenomenon where molecules with sufficient kinetic energy escape from the liquid's surface.
Example: Water boiling in a kettle. Heat from the kettle's element is absorbed by the water molecules, increasing their kinetic energy until they reach the boiling point. Further heat absorption overcomes the intermolecular forces, resulting in the formation of steam. Similarly, water evaporating from a puddle on a hot day absorbs energy from the sun and the surrounding air.
4. Condensation (Gas to Liquid) – Exothermic
Condensation is the opposite of vaporization. It involves the transition of a substance from a gaseous state to a liquid state. During condensation, gas molecules lose energy, reducing their kinetic energy. This allows the intermolecular forces to pull the molecules closer together, resulting in the formation of a liquid. The energy released during condensation is the energy that was previously absorbed during vaporization.
Example: Water vapor forming dew on a cold surface. As water vapor in the air comes into contact with a cooler surface, it loses energy, causing the molecules to slow down and condense into liquid water droplets. The energy released during condensation warms the surface slightly. Fog and cloud formation are also examples of condensation.
5. Sublimation (Solid to Gas) – Endothermic
Sublimation is the transition of a substance from a solid state directly to a gaseous state without passing through the liquid state. This process requires energy input to overcome the strong intermolecular forces holding the solid particles together, allowing them to escape directly into the gaseous phase.
Example: Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas. Dry ice absorbs energy from its surroundings, causing the CO2 molecules to transition directly from the solid to the gaseous phase without melting into a liquid. This is why dry ice doesn't leave puddles.
6. Deposition (Gas to Solid) – Exothermic
Deposition is the opposite of sublimation. It involves the transition of a substance from a gaseous state directly to a solid state without passing through the liquid state. During deposition, gas molecules lose energy, causing them to slow down and their kinetic energy to decrease. This allows the intermolecular forces to draw the particles closer together, forming a solid directly from the gas phase.
Example: Frost formation on cold surfaces. Water vapor in the air loses energy when it comes into contact with a cold surface, transitioning directly from a gaseous state to a solid state (ice) without forming liquid water. The energy released during deposition slightly warms the surface.
Factors Affecting Phase Changes
Several factors can influence the rate and temperature at which phase changes occur:
-
Temperature: Higher temperatures generally favor endothermic phase changes (melting, vaporization, sublimation), while lower temperatures favor exothermic phase changes (freezing, condensation, deposition).
-
Pressure: Pressure plays a significant role, especially in the liquid-gas equilibrium. Higher pressure generally favors the condensed phase (liquid or solid).
-
Intermolecular Forces: Strong intermolecular forces require more energy to overcome during endothermic phase changes and release more energy during exothermic phase changes. Substances with strong intermolecular forces will generally have higher melting and boiling points.
-
Impurities: The presence of impurities in a substance can affect its melting and boiling points, often causing them to be lower than those of the pure substance.
Real-World Applications
Understanding phase changes is crucial in various applications:
-
Weather: Weather patterns are heavily influenced by phase changes of water. Evaporation, condensation, precipitation, and sublimation are integral parts of the water cycle.
-
Industry: Many industrial processes rely on phase changes, such as distillation (separation of liquids based on boiling points), freeze-drying (preservation of food through sublimation), and refrigeration (cooling through the absorption of heat during vaporization).
-
Material Science: Phase changes are critical in material science for creating new materials with specific properties. Controlling the phase transitions allows for the development of alloys, polymers, and other advanced materials.
Conclusion
Phase changes are fundamental physical processes that involve the absorption or release of energy, classifying them as endothermic or exothermic. Melting, vaporization, and sublimation are endothermic processes, while freezing, condensation, and deposition are exothermic processes. Understanding these processes and the factors that affect them is essential in various scientific and technological fields, from meteorology and industrial processes to material science and everyday life. The energy changes involved are critical for understanding and manipulating the properties of matter. Further exploration of thermodynamics provides deeper insight into the energetic aspects of these fascinating transformations.
Latest Posts
Latest Posts
-
What Is The Opposite Of Sublimation
Mar 17, 2025
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Classify The Phase Changes As Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
