Can A Buffer Be Made With A Strong Acid
Muz Play
Mar 17, 2025 · 5 min read
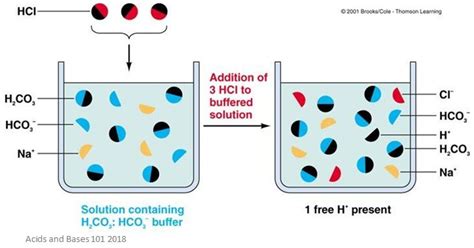
Table of Contents
Can a Buffer Be Made with a Strong Acid?
The short answer is: no, a buffer cannot be made using only a strong acid. This is a fundamental concept in chemistry, and understanding why is crucial for anyone working with solutions and pH control. This article will delve deep into the definition of a buffer, the properties of strong and weak acids and bases, and explain why a strong acid simply cannot form a buffer solution on its own. We'll also explore related concepts and offer practical examples to solidify your understanding.
Understanding Buffer Solutions
A buffer solution is an aqueous solution that resists changes in pH upon the addition of small amounts of acid or base. This resistance to pH change is its defining characteristic and what makes buffers so important in various applications, from biological systems to chemical processes. Buffers achieve this remarkable stability through the presence of a weak acid and its conjugate base, or a weak base and its conjugate acid. This equilibrium between the weak acid/base and its conjugate allows the system to absorb added H⁺ or OH⁻ ions without significant pH shifts.
The Key Role of Weak Acids and Bases
The magic of a buffer lies in the weak acid-base conjugate pair. A weak acid only partially dissociates in water, meaning it doesn't completely break down into its ions (H⁺ and its conjugate base). This creates an equilibrium where both the undissociated acid and its conjugate base are present in significant amounts. When a strong acid is added, the conjugate base reacts with the added H⁺ ions, minimizing the pH change. Similarly, when a strong base is added, the weak acid reacts with the added OH⁻ ions, again preventing a drastic pH shift.
This equilibrium is described by the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
where:
- pH is the solution's pH
- pKa is the negative logarithm of the acid dissociation constant (Ka) of the weak acid
- [A⁻] is the concentration of the conjugate base
- [HA] is the concentration of the weak acid
This equation highlights the critical role of the ratio of conjugate base to weak acid in determining the buffer's pH. A crucial aspect is that both [A⁻] and [HA] must be present in significant, comparable concentrations for effective buffering.
Why Strong Acids Cannot Form Buffers
Strong acids, unlike weak acids, completely dissociate in water. This means that when a strong acid like HCl is dissolved in water, it essentially exists entirely as H⁺ and Cl⁻ ions. There's no significant equilibrium between the undissociated acid and its conjugate base. There is no conjugate base to react with added base. The system lacks the inherent mechanism that allows buffers to resist pH changes.
Imagine trying to create a buffer with only HCl. Adding a base would neutralize the HCl, but there's nothing left to resist further pH changes. The buffer capacity, its ability to resist pH shifts, would be virtually nonexistent. Similarly, adding more HCl would just increase the acidity without any counteracting mechanism. This illustrates why a strong acid alone is unsuitable for buffer preparation.
Practical Examples and Applications
Let's compare creating a buffer with a weak acid and a strong acid:
Example 1: Acetic acid/acetate buffer
This classic example uses acetic acid (CH₃COOH), a weak acid, and its conjugate base, acetate (CH₃COO⁻). A solution containing both will resist pH changes. The Henderson-Hasselbalch equation can be used to calculate the required concentrations to achieve a desired pH.
Example 2: Attempted buffer with HCl
Trying to create a buffer with HCl alone would be futile. The addition of even a small amount of base would drastically change the pH, and there would be no mechanism to resist further changes. The solution would simply neutralize, lacking the buffering capacity of a weak acid-conjugate base system.
Buffer Capacity and pH Range
The effectiveness of a buffer isn't infinite. The buffer capacity refers to the amount of acid or base a buffer can neutralize before a significant pH change occurs. This capacity is highest when the concentrations of the weak acid and its conjugate base are roughly equal (i.e., when [A⁻]/[HA] ≈ 1 in the Henderson-Hasselbalch equation). The effective pH range of a buffer is typically within ±1 pH unit of the pKa of the weak acid.
Choosing the right weak acid for a buffer is crucial, as it dictates the pKa and therefore the effective pH range. Different applications require buffers with different pH ranges.
Importance of Buffers in Various Fields
Buffer solutions are essential across numerous scientific disciplines and industries. Here are a few key examples:
- Biology: Biological systems rely heavily on buffers to maintain a stable pH. Blood, for instance, employs the bicarbonate buffer system to maintain a pH around 7.4. This is crucial for enzyme activity and overall physiological function.
- Chemistry: Buffers are critical in many chemical reactions and processes where maintaining a constant pH is essential for optimal reaction yields or preventing unwanted side reactions.
- Medicine: Intravenous solutions often contain buffers to maintain the correct pH for compatibility with the body.
- Agriculture: Soil pH is critical for plant growth, and buffers can be used to adjust and stabilize soil pH.
- Industry: Many industrial processes rely on buffers to control pH in various stages of production.
Related Concepts: Polyprotic Acids and Buffers
Polyprotic acids can donate more than one proton. Each proton dissociation has its own Ka value, and different buffer systems can be created using various combinations of the polyprotic acid and its conjugate bases. For example, phosphoric acid (H₃PO₄) has three pKa values, allowing for buffers in different pH ranges.
Conclusion: The Irreplaceable Role of Weak Acids in Buffer Systems
In conclusion, creating a buffer requires a weak acid and its conjugate base (or a weak base and its conjugate acid). Strong acids, due to their complete dissociation in water, cannot form a buffer solution on their own. The absence of a significant equilibrium between the undissociated acid and its conjugate base eliminates the crucial mechanism for resisting pH changes. The properties of weak acids and their conjugate bases are fundamental to understanding and utilizing buffer solutions across diverse applications. Understanding buffer chemistry is essential for anyone involved in fields where pH control is paramount.
Latest Posts
Latest Posts
-
Does Water Go From High To Low Concentration
Mar 17, 2025
-
Circle Math Triangle Extending From Circle
Mar 17, 2025
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Can A Buffer Be Made With A Strong Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
