Does Water Go From High To Low Concentration
Muz Play
Mar 17, 2025 · 6 min read
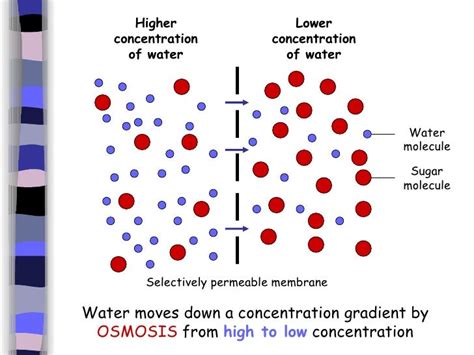
Table of Contents
Does Water Go From High to Low Concentration? Understanding Osmosis and Diffusion
The simple answer is: not always. While the movement of water is often associated with a concentration gradient—from high to low concentration—the reality is more nuanced. It depends heavily on the context, specifically whether we're discussing diffusion or osmosis. These two processes, while related, have distinct mechanisms and behaviors. Let's delve into the specifics to understand the complexities of water movement.
Diffusion: A Simple Movement Down the Concentration Gradient
Diffusion is the fundamental process where particles move from an area of high concentration to an area of low concentration. This movement continues until equilibrium is reached, meaning the concentration is uniform throughout the system. Think of a drop of food coloring in a glass of water. Initially, the dye is highly concentrated in one spot. Over time, the dye molecules spread out, diffusing throughout the water until the color is evenly distributed.
This principle applies to water molecules as well. If you have two containers separated by a permeable membrane, and one container has a higher concentration of water molecules (meaning less solute), water will diffuse across the membrane into the container with a lower concentration of water (higher solute concentration). This is a straightforward example of water moving down its concentration gradient.
Factors Affecting Diffusion Rate
Several factors influence the rate of diffusion:
- Temperature: Higher temperatures increase the kinetic energy of molecules, leading to faster diffusion.
- Concentration gradient: A steeper gradient (bigger difference in concentration) results in faster diffusion.
- Distance: The shorter the distance, the faster the diffusion.
- Size and mass of the molecules: Smaller and lighter molecules diffuse faster.
- Permeability of the membrane: A more permeable membrane allows for faster diffusion.
Osmosis: Water's Special Movement Across Semi-permeable Membranes
Osmosis is a specific type of diffusion that involves the movement of water across a semi-permeable membrane. A semi-permeable membrane allows the passage of water molecules but restricts the movement of larger solute molecules. This is crucial because it creates a situation where water movement is driven not solely by the total concentration of water, but by the water potential.
Water potential is a measure of the free energy of water. It's affected by several factors, including:
- Solute concentration: Higher solute concentration lowers water potential. Solutes bind water molecules, reducing the number of free water molecules available to move.
- Pressure: Pressure can increase or decrease water potential. Positive pressure increases water potential, while negative pressure (tension) decreases it.
- Gravity: Gravity can influence water potential, particularly in taller plants.
In osmosis, water moves from an area of high water potential to an area of low water potential. This means water moves across the semi-permeable membrane from a region of low solute concentration (high water potential) to a region of high solute concentration (low water potential). This movement continues until equilibrium is reached, or until a counteracting pressure prevents further water movement.
Osmotic Pressure
Osmotic pressure is the pressure required to prevent the movement of water across a semi-permeable membrane. It's a measure of the tendency of water to move into a solution by osmosis. A solution with a high solute concentration will have a high osmotic pressure because it exerts a strong pull on water molecules.
Types of Osmotic Solutions
To further understand osmosis, it's helpful to classify solutions based on their tonicity:
- Isotonic solution: The solute concentration is equal on both sides of the membrane. There's no net movement of water.
- Hypotonic solution: The solute concentration is lower outside the cell (higher water potential) compared to inside the cell. Water moves into the cell, potentially causing it to swell or burst (lyse).
- Hypertonic solution: The solute concentration is higher outside the cell (lower water potential) compared to inside the cell. Water moves out of the cell, causing it to shrink (crenate).
Water Movement in Biological Systems: A Complex Dance
In living organisms, water movement is rarely a simple diffusion process. It's largely governed by osmosis and is intricately linked to maintaining cellular integrity and overall homeostasis. Cell membranes are semi-permeable, allowing water to move freely while regulating the passage of other molecules. This precise control of water balance is crucial for cell function and survival.
Plant Cells: Turgor Pressure and Plasmolysis
Plant cells are excellent examples of osmosis in action. The cell wall provides structural support, preventing the cell from bursting in hypotonic conditions. When water enters the cell, it creates turgor pressure, which pushes the cell membrane against the cell wall, maintaining the cell's rigidity and contributing to overall plant structure. Conversely, in hypertonic conditions, water leaves the cell, causing plasmolysis – the shrinking of the cytoplasm away from the cell wall.
Animal Cells: Maintaining Fluid Balance
Animal cells lack a rigid cell wall, making them more vulnerable to osmotic changes. Maintaining fluid balance within and around animal cells is essential. Processes like active transport and ion channels play a role alongside osmosis in regulating the precise movement of water and other molecules across cell membranes. Dysregulation of these processes can lead to serious health consequences.
Beyond Simple Concentration Gradients: The Role of Other Factors
While the concentration gradient is a major driving force, it's not the only factor determining water movement. Other factors like pressure, gravity, and even the presence of specific membrane channels significantly influence the direction and rate of water flow.
For instance, in plants, the process of transpiration (water loss through leaves) creates a negative pressure (tension) in the xylem vessels, pulling water upwards from the roots against gravity. This is a complex interplay of osmosis, capillary action, and cohesive and adhesive forces. The movement of water here isn't solely driven by a concentration gradient but by the overall water potential gradient across the entire plant system.
Similarly, in the human body, water movement in the kidneys is highly regulated to maintain blood pressure and electrolyte balance. The processes involved are far more intricate than simple diffusion, encompassing filtration, reabsorption, and secretion, all of which are influenced by hormones and other physiological factors.
Conclusion: Water Movement is Context-Dependent
While water often moves from areas of high concentration to low concentration, particularly in simple diffusion scenarios, the more accurate and comprehensive view considers water potential. In osmosis, the driving force is the difference in water potential across a semi-permeable membrane, which is influenced by solute concentration, pressure, and other factors.
Understanding the intricacies of diffusion and osmosis is crucial for comprehending numerous biological processes and phenomena. It's essential to remember that water movement isn't always a simple case of following the concentration gradient but a complex interplay of several forces acting in concert. The context—whether it’s a simple beaker experiment or a complex biological system— fundamentally shapes how water moves.
Latest Posts
Latest Posts
-
Electrons Are Located In Energy Levels Called Electron
Mar 17, 2025
-
Can Mitochondria Survive Outside The Cell
Mar 17, 2025
-
Name A Structural Difference Between Triglycerides And Phospholipids
Mar 17, 2025
-
Ending Materials In A Chemical Reaction
Mar 17, 2025
-
Get Energy By Eating Other Organisms
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Water Go From High To Low Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
