Electrons Are Located In Energy Levels Called Electron
Muz Play
Mar 17, 2025 · 6 min read
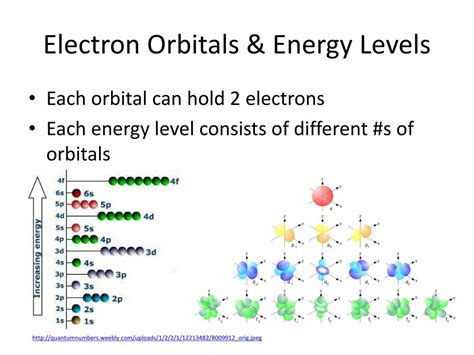
Table of Contents
Electrons are Located in Energy Levels Called Electron Shells: A Deep Dive into Atomic Structure
The atom, the fundamental building block of matter, is a fascinating microcosm of energy and structure. While often depicted as a miniature solar system, the reality of atomic structure is far more nuanced and governed by the principles of quantum mechanics. Central to understanding this structure is the concept of electron shells, energy levels where electrons reside, dictating an atom's properties and its interactions with other atoms. This article delves deep into the fascinating world of electron shells, exploring their significance, their relationship to electron configuration, and their implications in chemistry and physics.
Understanding Electron Shells: The Quantized World of Electrons
Electrons, negatively charged subatomic particles, don't orbit the nucleus in neat, predictable paths like planets around a star. Instead, their behavior is governed by the principles of quantum mechanics, meaning their positions and energies are probabilistic rather than deterministic. This means we can't pinpoint an electron's exact location at any given moment; instead, we describe its probable location within a region of space called an orbital.
These orbitals are grouped into electron shells, also known as energy levels. Each shell represents a distinct energy level, with electrons in lower shells possessing lower energy than those in higher shells. This energy difference is crucial in understanding chemical reactions and the stability of atoms. The closer an electron is to the nucleus, the stronger the electrostatic attraction and the lower its energy. As we move further from the nucleus to higher energy levels, this attraction weakens, and the energy of the electrons increases.
Principal Quantum Number (n) and Shell Designation
The energy level, or shell, of an electron is primarily determined by its principal quantum number (n). This number is always a positive integer (n = 1, 2, 3, ...), with n=1 representing the lowest energy level (closest to the nucleus) and increasing values of n corresponding to higher energy levels further from the nucleus. These shells are often designated by letters:
- n = 1: K shell
- n = 2: L shell
- n = 3: M shell
- n = 4: N shell
- n = 5: O shell
- n = 6: P shell
- n = 7: Q shell
Higher values of 'n' represent shells with higher energy and larger size, meaning the electrons are further from the nucleus and less tightly bound. This directly impacts an atom's reactivity and its ability to form chemical bonds.
Subshells and Orbitals: A Deeper Look into Electron Arrangement
Each electron shell, except for the first (K shell), is further divided into subshells. These subshells represent different regions of space within the shell where electrons are likely to be found and are characterized by their azimuthal quantum number (l). The value of 'l' can range from 0 to n-1, meaning that a shell with principal quantum number 'n' has 'n' subshells.
- l = 0: s subshell (spherical orbital)
- l = 1: p subshell (dumbbell-shaped orbitals)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
Each subshell contains a specific number of orbitals, which are regions of space where there's a high probability of finding an electron. The number of orbitals in a subshell is determined by the magnetic quantum number (ml), which can range from -l to +l, including 0. Therefore:
- s subshell: 1 orbital (holds up to 2 electrons)
- p subshell: 3 orbitals (holds up to 6 electrons)
- d subshell: 5 orbitals (holds up to 10 electrons)
- f subshell: 7 orbitals (holds up to 14 electrons)
It's important to understand that each orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms - the spin quantum number).
Electron Configuration and the Periodic Table
The arrangement of electrons within an atom's shells and subshells is called its electron configuration. This configuration determines the atom's chemical properties and its position in the periodic table. Atoms tend to fill their lower energy levels first, following the Aufbau principle (building-up principle), which dictates that electrons occupy the lowest available energy levels. The Hund's rule further specifies that electrons will individually occupy each orbital within a subshell before pairing up.
For example, let's consider the electron configuration of carbon (atomic number 6):
1s² 2s² 2p²
This indicates that carbon has:
- 2 electrons in the 1s orbital (K shell)
- 2 electrons in the 2s orbital (L shell)
- 2 electrons in the 2p orbitals (L shell)
The periodic table itself is a visual representation of electron configurations. Elements within the same group (column) have similar outer electron shell configurations, leading to similar chemical behaviors. The periodic trends in properties like electronegativity, ionization energy, and atomic radius are directly related to electron shell structure.
Significance of Electron Shells in Chemical Bonding
The outermost electron shell, called the valence shell, plays a crucial role in chemical bonding. Electrons in the valence shell are involved in forming chemical bonds with other atoms. Atoms tend to react in ways that achieve a stable electron configuration, often by gaining, losing, or sharing electrons to fill their valence shell. This drive towards stability is the foundation of chemical bonding and explains the formation of molecules and compounds.
Ionic Bonds: Electron Transfer
In ionic bonding, atoms transfer electrons to achieve a stable electron configuration. For example, sodium (Na) readily loses its single valence electron to become a positively charged ion (Na+), while chlorine (Cl) readily gains an electron to become a negatively charged ion (Cl−). The electrostatic attraction between these oppositely charged ions forms an ionic bond.
Covalent Bonds: Electron Sharing
In covalent bonding, atoms share electrons to achieve a stable electron configuration. For example, two hydrogen atoms share their single electrons to form a stable hydrogen molecule (H₂), where each hydrogen atom effectively achieves a filled valence shell.
Beyond the Basics: Excited States and Electron Transitions
Electrons typically occupy the lowest energy levels available, but they can absorb energy and move to higher energy levels, resulting in an excited state. This transition occurs when an electron absorbs a photon of light with energy equal to the energy difference between the two energy levels. The excited state is unstable, and the electron will eventually return to a lower energy level, emitting a photon of light in the process. This process is the basis of atomic spectroscopy, a powerful technique used to identify elements based on their characteristic emission and absorption spectra.
Conclusion: Electron Shells as the Foundation of Atomic Behavior
Electron shells are not simply abstract concepts; they are the fundamental framework that dictates the behavior of atoms and their interactions. Understanding the arrangement of electrons within these shells is crucial for comprehending the properties of elements, the formation of chemical bonds, and the intricacies of atomic spectroscopy. The principles discussed – quantum numbers, subshells, orbitals, and the various bonding mechanisms – form the cornerstone of modern chemistry and physics, providing a detailed explanation for the remarkable diversity and complexity of the material world around us. The exploration of electron shells remains a vibrant area of research, contributing to advancements in materials science, nanotechnology, and our fundamental understanding of the universe.
Latest Posts
Latest Posts
-
How To Calculate Saturated Vapour Pressure
Mar 17, 2025
-
Conversion From Cartesian To Cylindrical Coordinates
Mar 17, 2025
-
Is An Atom Smaller Than A Molecule
Mar 17, 2025
-
Why Does Gaining An Electron Give You A Negative Charge
Mar 17, 2025
-
Converting Rectangular Coordinates To Polar Coordinates
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Electrons Are Located In Energy Levels Called Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
