Is An Atom Smaller Than A Molecule
Muz Play
Mar 17, 2025 · 6 min read
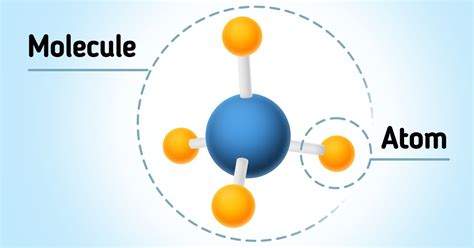
Table of Contents
Is an Atom Smaller Than a Molecule? A Deep Dive into the Building Blocks of Matter
The question, "Is an atom smaller than a molecule?" might seem simple at first glance. The answer, however, opens the door to a fascinating exploration of chemistry, physics, and the fundamental building blocks of our universe. Understanding the relationship between atoms and molecules is crucial for grasping the nature of matter and the behavior of the world around us. This comprehensive guide delves into the intricacies of atomic and molecular structure, explaining the size difference and the significance of this distinction in various scientific contexts.
Understanding Atoms: The Fundamental Units of Matter
Atoms are the basic units of matter that retain the chemical properties of an element. Think of them as the indivisible Lego bricks of the universe. While the ancient Greeks conceptualized the atom, it was only through the scientific advancements of the 19th and 20th centuries that we gained a deeper understanding of their structure.
The Subatomic Particles: Protons, Neutrons, and Electrons
Atoms are not solid, impenetrable spheres. Instead, they consist of a tiny, dense nucleus containing positively charged protons and electrically neutral neutrons. Surrounding this nucleus is a cloud of negatively charged electrons, which are significantly smaller and lighter than protons and neutrons. The number of protons in an atom's nucleus determines its atomic number and identifies the element. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon.
Atomic Size and Measurement: The Angstrom and Picometer
Atoms are incredibly small, far too small to be seen with even the most powerful optical microscopes. Their size is typically measured in angstroms (Å) or picometers (pm). One angstrom is equal to 0.1 nanometers (nm), and one picometer is one trillionth of a meter. Atomic radii (the distance from the nucleus to the outermost electron) vary depending on the element, typically ranging from about 50 to 300 pm. Hydrogen, the smallest atom, has a radius of approximately 53 pm.
Molecules: Aggregates of Atoms
Unlike atoms, which represent individual elements, molecules are formed when two or more atoms chemically bond together. These bonds are formed through the interactions of electrons in the outer shells of the atoms. The types of bonds—covalent, ionic, or metallic—determine the properties of the resulting molecule.
Covalent Bonds: Sharing Electrons
In covalent bonding, atoms share electrons to achieve a more stable electron configuration. This type of bonding is common in molecules composed of nonmetals, such as water (H₂O), methane (CH₄), and carbon dioxide (CO₂). The shared electrons create a strong attractive force that holds the atoms together.
Ionic Bonds: Transferring Electrons
Ionic bonding involves the transfer of electrons from one atom to another. This creates ions—charged atoms—with opposite charges that attract each other. This type of bond is common in compounds formed between metals and nonmetals, such as sodium chloride (NaCl), or table salt.
Metallic Bonds: A Sea of Electrons
In metallic bonding, valence electrons are delocalized and shared among a lattice of metal atoms. This explains the properties of metals like conductivity and malleability.
Molecular Size and Shape: A Wider Range of Variation
The size and shape of a molecule depend on the number and type of atoms it contains, as well as the types of bonds that hold them together. Molecules can range in size from small, simple diatomic molecules like oxygen (O₂) to large, complex macromolecules like proteins and DNA, containing thousands or even millions of atoms. The three-dimensional structure of a molecule plays a crucial role in determining its physical and chemical properties.
The Size Difference: Atoms are Significantly Smaller Than Molecules
Given that molecules are composed of multiple atoms, it logically follows that molecules are larger than the atoms they contain. The size difference can be substantial, depending on the complexity of the molecule. A water molecule (H₂O), for example, is larger than a single hydrogen atom or a single oxygen atom. The increase in size isn't simply additive; the interactions between the atoms contribute to the overall size and shape of the molecule.
Visualizing the Size Difference
While it's impossible to directly visualize atoms and molecules, analogies can help illustrate the size difference. Imagine an atom as a marble. A molecule would then be a structure built from several marbles, connected together in a specific arrangement. The more marbles (atoms) involved, the larger the structure (molecule) becomes.
Examples Illustrating the Size Difference
Let's consider some specific examples to highlight the disparity between atomic and molecular sizes:
-
Hydrogen molecule (H₂): This simple molecule is composed of two hydrogen atoms covalently bonded together. It's larger than a single hydrogen atom because the atoms are physically connected and occupy a larger volume.
-
Water molecule (H₂O): This molecule, essential for life, consists of two hydrogen atoms and one oxygen atom. It's significantly larger than either a hydrogen or oxygen atom. The bond angles and electron distribution contribute to its overall size and shape.
-
Glucose (C₆H₁₂O₆): This simple sugar molecule is composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. It's considerably larger than any of its constituent atoms, reflecting the complex arrangement of atoms within the molecule.
-
Proteins and DNA: These biological macromolecules are composed of thousands or millions of atoms. Their size is orders of magnitude larger than individual atoms and even relatively small molecules like glucose. Their complex three-dimensional structures are essential for their biological functions.
Implications of the Size Difference in Various Fields
The size difference between atoms and molecules has profound implications in various scientific fields:
Chemistry: Reactivity and Chemical Reactions
The size and structure of molecules directly influence their reactivity. Larger, more complex molecules may have more reactive sites, leading to more complex chemical reactions. The shape of a molecule plays a crucial role in determining whether it can fit into an enzyme's active site, for example.
Physics: Material Properties
The arrangement of atoms and molecules dictates the macroscopic properties of materials. For example, the strong covalent bonds in diamond lead to its hardness, while the weaker intermolecular forces in water contribute to its fluidity. Nanotechnology manipulates the properties of materials by controlling the arrangement of atoms and molecules at a nanoscale level.
Biology: Life Processes
The size and shape of biological molecules are essential for life processes. Proteins fold into specific three-dimensional shapes to perform their functions, while DNA's double helix structure allows for efficient storage and replication of genetic information. Understanding the interplay between atomic and molecular structures is fundamental to understanding biological systems.
Conclusion: A Fundamental Distinction with Far-Reaching Consequences
In summary, the answer to the question "Is an atom smaller than a molecule?" is a resounding yes. Atoms are the fundamental building blocks of matter, while molecules are aggregates of atoms held together by chemical bonds. The size difference is significant, and it has profound implications across multiple scientific disciplines. Understanding this fundamental distinction is key to unlocking the secrets of matter, from the smallest atomic particles to the largest biological macromolecules and beyond. The world we observe and interact with is a direct consequence of this fundamental size difference and the intricate interactions between atoms and molecules. Continuing research at the atomic and molecular levels promises to unveil further insights into the universe's complexities.
Latest Posts
Latest Posts
-
What Are The 3 Parts Of An Rna Nucleotide
Mar 18, 2025
-
When Do You Use Parentheses In Writing A Chemical Formula
Mar 18, 2025
-
Which Process Takes Place In Chloroplasts
Mar 18, 2025
-
How To Do A Slope Field
Mar 18, 2025
-
How Many Electrons Can The D Orbital Hold
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is An Atom Smaller Than A Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
