How Many Electrons Can The D Orbital Hold
Muz Play
Mar 18, 2025 · 5 min read
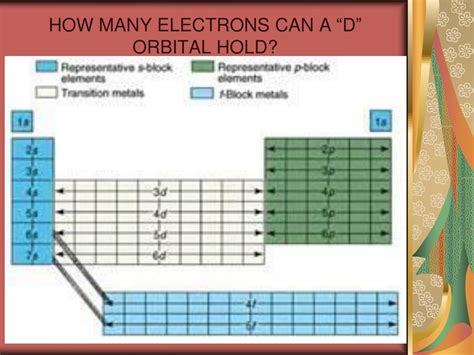
Table of Contents
How Many Electrons Can the d Orbital Hold? A Deep Dive into Atomic Structure
The question of how many electrons a d orbital can hold is fundamental to understanding atomic structure and the periodic table. While the simple answer is ten, a deeper exploration reveals the intricacies of quantum mechanics and the fascinating world of electron configuration. This article will delve into the specifics, exploring the underlying principles, clarifying common misconceptions, and demonstrating the significance of this concept in chemistry and beyond.
Understanding Atomic Orbitals
Before diving into the d orbital's electron capacity, let's establish a foundational understanding of atomic orbitals. According to quantum mechanics, electrons don't orbit the nucleus in well-defined paths like planets around a star. Instead, they exist in regions of space called atomic orbitals, which describe the probability of finding an electron at a particular location. These orbitals are characterized by a set of quantum numbers:
-
Principal Quantum Number (n): Determines the energy level and size of the orbital. n can be any positive integer (1, 2, 3...). Higher n values indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): Determines the shape of the orbital and its angular momentum. l can range from 0 to n-1. Different values of l correspond to different orbital types:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): Specifies the orientation of the orbital in space. ml can range from -l to +l, including 0. This means:
- s orbital: 1 orientation (ml = 0)
- p orbital: 3 orientations (ml = -1, 0, +1)
- d orbital: 5 orientations (ml = -2, -1, 0, +1, +2)
- f orbital: 7 orientations (ml = -3, -2, -1, 0, +1, +2, +3)
-
Spin Quantum Number (ms): Describes the intrinsic angular momentum of the electron, often visualized as "spin up" (+1/2) or "spin down" (-1/2). This is crucial for understanding the Pauli Exclusion Principle.
The Pauli Exclusion Principle and Electron Capacity
The Pauli Exclusion Principle is a cornerstone of quantum mechanics. It states that no two electrons in an atom can have the same set of four quantum numbers. This principle directly impacts the maximum number of electrons that can occupy an orbital.
Since each orbital is defined by n, l, and ml, and each electron can have one of two spin states (ms = +1/2 or -1/2), the maximum number of electrons an orbital can hold is two.
The Five d Orbitals and Their Electron Capacity
Now we can address the d orbital specifically. Because the azimuthal quantum number for a d orbital is l = 2, the magnetic quantum number (ml) can have five values: -2, -1, 0, +1, +2. This means there are five different d orbitals within a given energy level (e.g., 3d, 4d, 5d).
Each of these five d orbitals can hold a maximum of two electrons (one spin up and one spin down), according to the Pauli Exclusion Principle. Therefore, the total number of electrons a d subshell (which contains all five d orbitals) can hold is 5 orbitals * 2 electrons/orbital = 10 electrons.
Visualizing the d Orbitals
While visualizing the shapes of d orbitals can be challenging, understanding their spatial arrangement is key to grasping their electron capacity. The five d orbitals have complex shapes, often described as cloverleaf or donut-shaped with varying orientations in three-dimensional space. Their orientations are defined by their magnetic quantum numbers.
Implications in Chemistry and Beyond
The ability of a d orbital to hold ten electrons has profound implications in various fields, most notably in chemistry:
-
Transition Metals: Transition metals are characterized by partially filled d orbitals in their valence shells. This partially filled d shell is responsible for the diverse and often colorful chemistry exhibited by these elements. Their variable oxidation states, catalytic activity, and magnetic properties are all consequences of the d electrons' ability to participate in chemical bonding.
-
Coordination Complexes: Transition metal ions frequently form coordination complexes, where the metal ion is surrounded by ligands (molecules or ions). The d orbitals play a central role in the bonding within these complexes, influencing their geometry, stability, and reactivity. The ligand field theory explains how the energy levels of d orbitals are affected by the surrounding ligands.
-
Spectroscopy: The electronic transitions between different d orbitals are responsible for the characteristic absorption and emission spectra of transition metal compounds. This is exploited in various spectroscopic techniques used for identification and analysis.
-
Materials Science: The electronic structure, particularly the d orbitals, is critical in determining the properties of materials. For example, the magnetic properties of many materials arise from the presence of unpaired electrons in d orbitals.
Common Misconceptions
Several misconceptions surround the d orbital's electron capacity:
-
Confusing orbitals and shells: It's important to distinguish between an orbital (a region of space where an electron is likely to be found) and a shell (an energy level containing multiple orbitals). A shell can contain many orbitals, and each orbital can hold a maximum of two electrons.
-
Ignoring the Pauli Exclusion Principle: Failing to account for the Pauli Exclusion Principle leads to an incorrect electron capacity for any orbital, including the d orbital.
-
Oversimplifying electron configuration: Electron configuration is more complex than simply filling orbitals sequentially. Hund's rule dictates that electrons will individually occupy orbitals within a subshell before pairing up.
Conclusion
In conclusion, a d orbital can hold a maximum of two electrons due to the Pauli Exclusion Principle. However, a d subshell, containing five d orbitals, can accommodate a total of ten electrons. This seemingly simple fact has far-reaching consequences in various scientific disciplines, particularly in understanding the behavior of transition metals and their compounds. A thorough understanding of atomic orbitals, quantum numbers, and the principles governing electron configuration is fundamental to grasping the complexity and beauty of the atomic world. Further exploration into ligand field theory and advanced spectroscopic techniques will reveal even greater depths to the fascinating role of d orbitals in chemistry and materials science.
Latest Posts
Latest Posts
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The D Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
