Why Does Gaining An Electron Give You A Negative Charge
Muz Play
Mar 17, 2025 · 6 min read
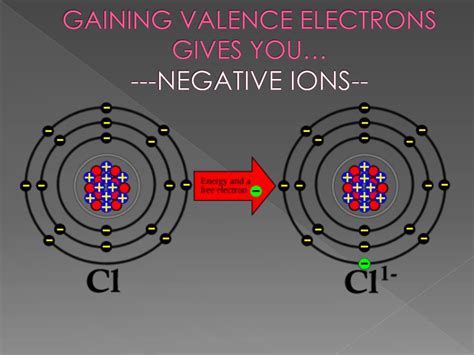
Table of Contents
Why Does Gaining an Electron Give You a Negative Charge? Understanding Atomic Structure and Charge
The seemingly simple question, "Why does gaining an electron give you a negative charge?" delves deep into the fundamental principles of atomic structure and the nature of electric charge. Understanding this requires exploring the building blocks of matter, the roles of protons, electrons, and neutrons, and how these particles interact to create the overall charge of an atom or ion. This article will provide a comprehensive explanation, suitable for both beginners and those seeking a deeper understanding of this key concept in chemistry and physics.
The Fundamental Particles: Protons, Neutrons, and Electrons
All matter is composed of atoms, and atoms themselves are made up of three primary subatomic particles:
-
Protons: These particles carry a positive charge (+1). They reside within the atom's nucleus, a dense central region. The number of protons in an atom's nucleus determines the element's atomic number and defines its identity. For instance, hydrogen has one proton, helium has two, and so on.
-
Neutrons: Neutrons are found in the nucleus alongside protons. As their name suggests, they carry no charge (0). Their primary role is to contribute to the nucleus's mass and stability. The number of neutrons can vary within the same element, leading to isotopes.
-
Electrons: These particles carry a negative charge (-1). They are significantly smaller and lighter than protons and neutrons and orbit the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom, resulting in a net charge of zero.
The Role of Electrons in Determining Charge
The key to understanding why gaining an electron results in a negative charge lies in the electron's inherent negative charge and the concept of charge balance. A neutral atom has an equal number of protons and electrons. The positive charges of the protons are perfectly balanced by the negative charges of the electrons, resulting in a net charge of zero.
However, atoms can gain or lose electrons, a process known as ionization. This alteration in the electron count disrupts the charge balance, leading to the formation of ions:
-
Anions: When an atom gains one or more electrons, it acquires a net negative charge because the number of negatively charged electrons now exceeds the number of positively charged protons. These negatively charged ions are called anions. For example, a chlorine atom (Cl) can gain one electron to become a chloride ion (Cl⁻), possessing a -1 charge.
-
Cations: Conversely, when an atom loses one or more electrons, it becomes positively charged because the number of protons now outweighs the number of electrons. These positively charged ions are called cations. For example, a sodium atom (Na) can lose one electron to become a sodium ion (Na⁺), possessing a +1 charge.
Why Negative? The Arbitrary Nature of Charge Assignment
The assignment of negative charge to the electron is, to a certain extent, arbitrary. It was a historical convention established during the early investigations into electricity. Scientists observed two types of charges—one attracting and the other repelling—and arbitrarily labeled one "positive" and the other "negative." Benjamin Franklin, a prominent figure in early electrical studies, made this distinction, and his convention remains in use today.
It's crucial to understand that the "negative" label doesn't imply a deficiency or lack of something. It simply indicates the type of charge the electron carries, which is opposite to the charge of the proton. The opposite charges are what leads to the attractive force between them, holding the atom together.
The Electromagnetic Force and Coulomb's Law
The interaction between charged particles is governed by the electromagnetic force, one of the four fundamental forces of nature. This force dictates how charged particles attract or repel each other. Coulomb's Law quantifies this interaction:
F = k * |q₁q₂| / r²
Where:
- F represents the force between the charges.
- k is Coulomb's constant.
- q₁ and q₂ are the magnitudes of the two charges.
- r is the distance between the charges.
This equation shows that the force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. Like charges (both positive or both negative) repel each other, while opposite charges (positive and negative) attract.
The attractive force between the positively charged protons in the nucleus and the negatively charged electrons is crucial for maintaining the atom's structure. The electrons are bound to the nucleus due to this electromagnetic interaction.
Gaining Electrons and Chemical Reactions
The process of gaining electrons is frequently observed in chemical reactions, especially in redox (reduction-oxidation) reactions. Reduction is the process where an atom gains electrons, and oxidation is the process where an atom loses electrons. These reactions are fundamental to many chemical processes, from corrosion to the functioning of batteries.
Consider the reaction between sodium (Na) and chlorine (Cl):
2Na + Cl₂ → 2NaCl
In this reaction, sodium atoms lose one electron each (oxidation), becoming Na⁺ ions, while chlorine atoms gain one electron each (reduction), becoming Cl⁻ ions. The resulting electrostatic attraction between the oppositely charged ions forms the ionic compound sodium chloride (NaCl), or common table salt.
Applications and Examples
The principle of gaining electrons and acquiring a negative charge has numerous applications and implications across various fields:
-
Electrochemistry: Batteries and fuel cells rely on the transfer of electrons between different materials to generate electrical energy. The movement of electrons and the formation of ions are central to their operation.
-
Materials Science: The electrical conductivity of materials is heavily influenced by the ability of electrons to move freely. Materials with loosely bound electrons tend to be good conductors, while those with tightly bound electrons are often insulators.
-
Biology: Many biological processes depend on electron transfer. For example, cellular respiration involves the transfer of electrons along the electron transport chain to generate ATP, the cell's energy currency.
-
Environmental Science: Understanding electron transfer is crucial in comprehending processes like corrosion, where metals lose electrons and react with oxygen and water.
Conclusion
In summary, gaining an electron gives an atom a negative charge because electrons inherently carry a negative charge. When an atom gains an electron, the balance of positive and negative charges within the atom is disrupted, resulting in a net negative charge. This fundamental concept, governed by the electromagnetic force and described by Coulomb's Law, is pivotal in understanding various chemical and physical phenomena and has broad applications in numerous scientific fields. The seemingly simple question of why gaining an electron leads to a negative charge opens the door to a profound understanding of the intricate world of atomic structure and the behavior of matter.
Latest Posts
Latest Posts
-
Which Process Takes Place In Chloroplasts
Mar 18, 2025
-
How To Do A Slope Field
Mar 18, 2025
-
How Many Electrons Can The D Orbital Hold
Mar 18, 2025
-
What Type Of Ion Do Metals Form
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Why Does Gaining An Electron Give You A Negative Charge . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
