Element Vs Compound Vs Homogeneous Vs Heterogeneous
Muz Play
Mar 17, 2025 · 5 min read
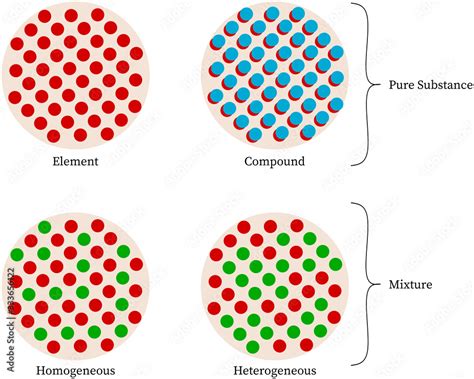
Table of Contents
Element vs. Compound vs. Homogeneous vs. Heterogeneous: Understanding the Building Blocks of Matter
Chemistry, at its core, is the study of matter and its transformations. Understanding the fundamental classifications of matter is crucial for grasping more complex chemical concepts. This article delves into the key differences between elements, compounds, homogeneous mixtures, and heterogeneous mixtures, clarifying their properties and providing examples to solidify your understanding. We'll explore their distinct characteristics, emphasizing the crucial distinctions that define each category. This comprehensive guide will equip you with a solid foundation in classifying matter, paving the way for a deeper exploration of the chemical world.
What is an Element?
An element is a pure substance consisting entirely of one type of atom. Atoms are the fundamental building blocks of matter, possessing a unique number of protons in their nucleus, known as the atomic number. This atomic number defines the element. Elements cannot be broken down into simpler substances by chemical means. The periodic table organizes all known elements based on their atomic number and recurring chemical properties.
Examples of Elements:
- Oxygen (O): A vital gas essential for respiration and combustion.
- Hydrogen (H): The lightest element, a crucial component of water and many organic compounds.
- Gold (Au): A highly valued precious metal known for its inertness and malleability.
- Iron (Fe): A strong, abundant metal widely used in construction and manufacturing.
- Carbon (C): The basis of organic chemistry, forming the backbone of countless molecules, from simple hydrocarbons to complex biomolecules like DNA.
What is a Compound?
A compound is a pure substance formed when two or more different elements chemically combine in a fixed ratio. This combination involves the formation of chemical bonds, creating a new substance with properties distinct from its constituent elements. Compounds can only be separated into their constituent elements through chemical processes, such as electrolysis or chemical reactions.
Characteristics of Compounds:
- Fixed composition: The ratio of elements in a compound is always constant.
- New properties: The properties of a compound are different from the properties of the elements that make it up. For example, sodium (a highly reactive metal) and chlorine (a toxic gas) combine to form sodium chloride (table salt), a relatively inert and edible compound.
- Chemical bonding: Compounds are held together by strong chemical bonds, such as ionic or covalent bonds.
Examples of Compounds:
- Water (H₂O): Composed of two hydrogen atoms and one oxygen atom, essential for life.
- Carbon dioxide (CO₂): A greenhouse gas produced by respiration and combustion.
- Sodium chloride (NaCl): Table salt, an ionic compound crucial for biological processes.
- Glucose (C₆H₁₂O₆): A simple sugar, a vital source of energy for living organisms.
- Sulfuric acid (H₂SO₄): A strong corrosive acid used in various industrial processes.
The Difference Between Elements and Compounds: A Clear Distinction
The fundamental distinction between elements and compounds lies in their composition and the nature of their constituents. Elements are composed of only one type of atom, while compounds are composed of two or more different types of atoms chemically bonded together. This chemical bonding fundamentally alters the properties of the constituent elements, resulting in a substance with unique characteristics. Elements cannot be broken down by chemical means, whereas compounds can be separated into their constituent elements through chemical reactions.
What is a Mixture?
A mixture is a physical combination of two or more substances that are not chemically bonded. The components of a mixture retain their individual properties, and their proportions can vary. Mixtures can be separated into their components by physical methods, such as filtration, distillation, or evaporation. Mixtures are further categorized into homogeneous and heterogeneous mixtures based on the uniformity of their composition.
What is a Homogeneous Mixture?
A homogeneous mixture has a uniform composition throughout. At the macroscopic level, you cannot distinguish the individual components. The mixture appears visually uniform, regardless of the sample size. Solutions are classic examples of homogeneous mixtures.
Characteristics of Homogeneous Mixtures:
- Uniform composition: The components are evenly distributed throughout the mixture.
- Single phase: Homogeneous mixtures exist in a single phase (solid, liquid, or gas).
- Invisible components: The individual components are not visibly distinguishable.
Examples of Homogeneous Mixtures:
- Air: A mixture of gases, primarily nitrogen, oxygen, and argon.
- Saltwater: A solution of salt dissolved in water.
- Sugar dissolved in water: A clear, uniform solution.
- Brass: An alloy of copper and zinc.
- Steel: An alloy of iron and carbon.
What is a Heterogeneous Mixture?
A heterogeneous mixture does not have a uniform composition throughout. The components are visibly distinguishable, and their proportions may vary from one part of the mixture to another. The different components exist in separate phases.
Characteristics of Heterogeneous Mixtures:
- Non-uniform composition: The components are not evenly distributed.
- Multiple phases: Heterogeneous mixtures often contain multiple phases (solid, liquid, or gas).
- Visible components: The individual components are visibly distinguishable.
Examples of Heterogeneous Mixtures:
- Sand and water: The sand particles are clearly visible and separate from the water.
- Oil and water: The oil and water layers separate, forming distinct phases.
- Soil: A complex mixture of minerals, organic matter, and water.
- Granite: A rock containing visibly different minerals, such as quartz, feldspar, and mica.
- A salad: A mixture of various vegetables and other ingredients.
The Key Differences Between Homogeneous and Heterogeneous Mixtures: A Comparative Analysis
The crucial distinction between homogeneous and heterogeneous mixtures lies in the uniformity of their composition. Homogeneous mixtures have a uniform composition throughout, whereas heterogeneous mixtures do not. This difference leads to observable variations in the appearance and properties of the mixtures. Homogeneous mixtures exhibit a single phase, while heterogeneous mixtures often consist of multiple phases, with distinct regions of different compositions.
Putting it All Together: A Comprehensive Overview
This detailed analysis helps solidify the understanding of the fundamental classifications of matter: elements, compounds, and mixtures (both homogeneous and heterogeneous). By recognizing the distinct characteristics of each category – from the atomic composition of elements and compounds to the uniform versus non-uniform nature of mixtures – we can effectively categorize various substances and predict their behavior. This foundational knowledge serves as a cornerstone for further exploration within the vast and intricate world of chemistry. Remembering the key differences between these categories will significantly enhance your understanding of chemical reactions, properties of substances, and the fundamental building blocks that constitute the material universe around us. The ability to distinguish between these classifications is essential for anyone seeking a deeper comprehension of chemical principles and their applications.
Latest Posts
Latest Posts
-
Difference Between Column And Thin Layer Chromatography
Mar 17, 2025
-
Opening A 6 Membered Ring Mechanism
Mar 17, 2025
-
What Is The Base Of A Triangle
Mar 17, 2025
-
Electrons Are Located In Energy Levels Called Electron
Mar 17, 2025
-
Can Mitochondria Survive Outside The Cell
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Element Vs Compound Vs Homogeneous Vs Heterogeneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
