Color Of Flame Of Calcium Chloride
Muz Play
Mar 28, 2025 · 6 min read
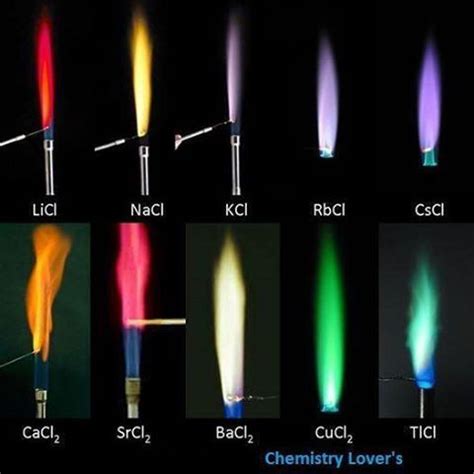
Table of Contents
The Enigmatic Flame Color of Calcium Chloride: A Deep Dive
The vibrant hues of flames have captivated humanity for centuries, from the flickering candles of ancient civilizations to the controlled burns of modern laboratories. Understanding the science behind these colors allows us to unlock a deeper understanding of the elemental composition of substances. One such substance that presents a fascinating case study is calcium chloride (CaCl₂), and its characteristic flame color is a subject worthy of detailed exploration. This article will delve into the specifics of calcium chloride's flame test, exploring the underlying physics and chemistry, common applications, potential safety concerns, and factors that can influence the observed color.
The Fundamentals: Atomic Structure and Flame Tests
Before diving into the specifics of calcium chloride, it's crucial to understand the fundamental principles governing flame color. When a metal salt, like calcium chloride, is introduced into a flame, the high temperature causes its atoms to become excited. This excitation involves electrons jumping to higher energy levels within the atom's electron shells. However, these higher energy levels are unstable. The electrons quickly fall back to their original, lower energy levels. This transition releases energy in the form of photons – tiny packets of light – whose wavelengths determine the color we perceive.
Each element possesses a unique electron configuration, resulting in a unique set of energy level transitions. This means each element emits light of specific wavelengths, creating a characteristic flame color. This is the principle behind the flame test, a qualitative analytical technique used in chemistry to identify unknown metal ions based on the color they impart to a flame.
The Fiery Orange-Red of Calcium: Deconstructing the Color
Calcium chloride, when subjected to a flame test, produces a characteristic brick-red or orange-red flame. This distinct color arises from the specific electronic transitions within the calcium atom (Ca). The electrons in calcium's outer shell absorb energy from the flame, jumping to higher energy levels. As they return to their ground state, they emit photons in the orange-red region of the visible light spectrum.
The precise shade of orange-red can vary slightly depending on several factors, including the concentration of the calcium chloride solution, the temperature of the flame, and the presence of other elements. These variations can lead to nuances in the observed color, ranging from a bright, fiery orange to a deeper, more brick-like red.
The Role of Electron Transitions: A Closer Look
The orange-red color specifically originates from the electron transitions within the calcium atom. The most prominent transitions involve the valence electrons in the 4s orbital. The energy difference between these energy levels corresponds to the wavelength of light in the orange-red part of the visible spectrum (approximately 600-700 nm). This is a fundamental principle in atomic spectroscopy, a powerful tool for analyzing the composition of matter.
Performing the Flame Test: A Practical Guide
While professional laboratories use advanced spectrometers for precise analysis, a simple flame test can be performed as a demonstrative experiment. The process is relatively straightforward:
-
Prepare a Solution: Dissolve a small amount of calcium chloride in distilled water. Using distilled water is important to avoid interference from other metal ions present in tap water.
-
Clean a Wire Loop: A clean nichrome or platinum wire loop is essential. Clean it thoroughly by dipping it in concentrated hydrochloric acid (HCl) and then flaming it until no color is observed. This removes any residual contaminants that might interfere with the test.
-
Dip and Flame: Dip the clean wire loop into the calcium chloride solution, ensuring a small amount of solution adheres to the loop.
-
Observe the Flame: Introduce the wire loop into a Bunsen burner flame. The flame should immediately turn a characteristic orange-red color.
-
Repeat and Compare: For comparison, it's beneficial to perform the flame test with solutions containing other metal salts to observe the differences in flame colors.
Important Note: Always exercise caution when working with Bunsen burners and chemicals. Ensure proper ventilation and wear appropriate safety gear, such as safety goggles.
Factors Affecting Flame Color: Nuances and Variations
While the orange-red color is characteristic of calcium, several factors can subtly influence the observed flame color:
-
Concentration: Higher concentrations of calcium chloride generally produce a more intense orange-red color. Dilute solutions might produce a fainter color, or even be difficult to observe.
-
Flame Temperature: The temperature of the flame also affects the intensity of the color. A hotter flame will generally excite more calcium atoms, resulting in a brighter, more vibrant color.
-
Presence of Other Ions: If other metal ions are present in the solution, they might interfere with the observed color. This can lead to a mixture of colors, obscuring the characteristic orange-red of calcium. This is why using distilled water is crucial.
-
Presence of Chloride Ions: While the calcium ion is primarily responsible for the color, the chloride ions (Cl⁻) also play a small role, possibly influencing the overall intensity and shade of the color. However, this influence is secondary to the calcium ions' contribution.
Calcium Chloride: Applications Beyond the Flame Test
Beyond its role in flame tests, calcium chloride finds numerous practical applications across various industries:
-
De-icing Agent: This is perhaps its most well-known application. Calcium chloride's ability to lower the freezing point of water makes it an effective de-icer for roads and pavements during winter.
-
Desiccant: Its hygroscopic nature makes it a useful desiccant, absorbing moisture from the air. This property is utilized in many industrial processes and products.
-
Food Additive: In the food industry, calcium chloride is used as a firming agent and a nutritional supplement, providing calcium.
-
Construction: Calcium chloride is employed in concrete mixes to accelerate setting and increase strength.
-
Medical Applications: It has certain medical applications, though this should only be under strict medical supervision.
Safety Considerations: Handling Calcium Chloride Responsibly
While calcium chloride is generally considered a safe compound, it is important to handle it responsibly:
-
Avoid Inhalation: Avoid inhaling calcium chloride dust, as it can irritate the respiratory system.
-
Eye Protection: Always wear safety goggles when handling calcium chloride, as contact with the eyes can cause irritation.
-
Skin Contact: If calcium chloride comes into contact with skin, wash the area thoroughly with water.
-
Storage: Store calcium chloride in a cool, dry place, away from moisture and incompatible materials.
Conclusion: The Enduring Significance of Flame Color
The orange-red flame color produced by calcium chloride is a testament to the fundamental principles of atomic structure and spectroscopy. Understanding this color, and the processes that give rise to it, allows us to appreciate the power of flame tests as a simple yet effective method for identifying elements. Moreover, exploring the diverse applications of calcium chloride, coupled with understanding its handling precautions, underlines the practical significance of this common compound. Through continued research and investigation, our understanding of the fascinating world of flame colors, and their underlying chemical mechanisms, will continue to expand.
Latest Posts
Latest Posts
-
Keratin And Collagen Are Examples Of Which Class Of Proteins
Mar 31, 2025
-
Job Order Costing Vs Process Costing
Mar 31, 2025
-
What Is The Chemical Equation For Aerobic Respiration
Mar 31, 2025
-
Equation Of The Tangent Line Implicit Differentiation
Mar 31, 2025
-
What Is The Secondary Structure Of Dna
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Color Of Flame Of Calcium Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
