Compared With Solid Ionic Compounds Solid Molecular Compounds Generally
Muz Play
Mar 22, 2025 · 6 min read
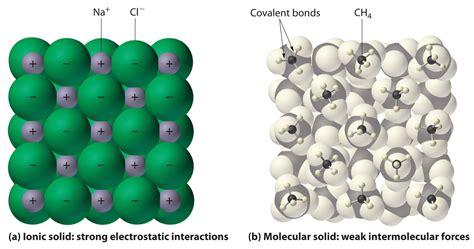
Table of Contents
Compared with Solid Ionic Compounds, Solid Molecular Compounds Generally... Exhibit Different Properties
Solid ionic and molecular compounds, while both forming solid structures, exhibit vastly different properties stemming from fundamental differences in their bonding and structure. Understanding these differences is crucial in various fields, from materials science and chemistry to pharmaceuticals and geology. This article delves into a detailed comparison of solid ionic and molecular compounds, highlighting their contrasting characteristics.
Bonding: The Foundation of Difference
The core distinction lies in the type of bonding present:
Ionic Compounds: The Electrostatic Embrace
Ionic compounds are formed through the electrostatic attraction between oppositely charged ions. This occurs when atoms with significantly different electronegativities interact. Highly electronegative atoms, like halogens and oxygen, readily gain electrons to form negatively charged anions, while less electronegative atoms, such as alkali and alkaline earth metals, readily lose electrons to form positively charged cations. The resulting electrostatic force between these ions is remarkably strong, leading to the formation of a crystalline lattice structure. The strength of this bond is directly related to the magnitude of the charges and the distance between the ions – Coulomb's Law governs this interaction. Examples include sodium chloride (NaCl), potassium iodide (KI), and magnesium oxide (MgO).
Molecular Compounds: The Covalent Connection
Molecular compounds, in contrast, are formed through covalent bonds. In covalent bonding, atoms share electrons to achieve a stable electron configuration. This sharing results in a relatively stronger bond compared to intermolecular forces, but typically weaker than the electrostatic attraction in ionic compounds. The shared electrons are attracted to the nuclei of both atoms, creating a stable molecular unit. The strength of a covalent bond depends on the degree of electron sharing and the size and electronegativity of the atoms involved. Water (H₂O), carbon dioxide (CO₂), and glucose (C₆H₁₂O₆) are classic examples of molecular compounds.
Structure: A Tale of Two Lattices
The type of bonding directly influences the resulting crystal lattice structure:
The Ordered Array of Ions: Ionic Crystalline Structure
Ionic compounds typically form crystalline lattices, characterized by a highly ordered, three-dimensional arrangement of ions. The arrangement is determined by the relative sizes and charges of the ions involved, aiming to maximize electrostatic attraction and minimize repulsion. This ordered structure contributes significantly to the physical properties of ionic compounds. The strong electrostatic forces extend throughout the entire lattice, making the structure very stable. The repeating pattern forms a unit cell which can be described by various crystal systems (cubic, tetragonal, orthorhombic, etc.). This structure explains several of their physical properties discussed later.
The Discrete Molecular Units: Molecular Crystalline Structure
Molecular compounds, on the other hand, consist of discrete molecules held together by intermolecular forces. These forces are considerably weaker than the ionic bonds in ionic compounds. Although they also often exhibit a crystalline structure, the arrangement is based on the interaction between entire molecules, rather than individual ions. The molecules themselves are held together by strong covalent bonds, but the forces between the molecules are much weaker, leading to significantly different properties. The types of intermolecular forces—van der Waals forces, dipole-dipole interactions, and hydrogen bonding—influence the arrangement and strength of the crystalline structure.
Physical Properties: A Clear Contrast
The differences in bonding and structure lead to marked differences in physical properties:
Melting and Boiling Points: A Measure of Bond Strength
Ionic compounds generally have much higher melting and boiling points than molecular compounds. The strong electrostatic forces in ionic lattices require a substantial amount of energy to overcome, resulting in high melting and boiling points. Molecular compounds, with their weaker intermolecular forces, require significantly less energy to break apart, leading to lower melting and boiling points.
Solubility: The Role of Polarity and Intermolecular Forces
Solubility in water also differs significantly. Many ionic compounds are soluble in water because water molecules, being polar, can effectively interact with and surround the charged ions, thus breaking the ionic bonds. The solubility of ionic compounds can vary depending on the specific ions involved and the strength of the ionic bonds. Molecular compounds' solubility depends on their polarity and the ability to form hydrogen bonds or other intermolecular interactions with water molecules. Polar molecular compounds often dissolve in water, while nonpolar ones tend to be insoluble.
Electrical Conductivity: Ions vs. Molecules
Electrical conductivity is another key difference. Ionic compounds are generally good conductors of electricity when molten or dissolved in water, as the ions become mobile and can carry an electric current. In the solid state, however, the ions are fixed in their lattice positions and cannot move freely, hence solid ionic compounds do not conduct electricity. Molecular compounds, in contrast, are generally poor conductors of electricity in both solid and liquid states, as they lack mobile charged particles. Exceptions exist, such as aqueous solutions of strong acids and bases, which conduct due to the presence of ions formed upon dissociation.
Hardness and Brittleness: Structure and Fracture
Ionic compounds are often hard and brittle. The strong electrostatic forces holding the ions together contribute to their hardness. However, their brittleness arises from the rigid structure. Applying force can cause the layers of ions to shift, leading to repulsion between ions of like charges, causing the crystal to fracture. Molecular compounds, depending on the intermolecular forces present, can exhibit varying degrees of hardness and brittleness. They are often softer and less brittle than ionic compounds.
Volatility: The Ease of Vaporization
Molecular compounds generally exhibit higher volatility than ionic compounds. Volatility refers to the ease with which a substance vaporizes. The weaker intermolecular forces in molecular compounds require less energy to overcome, resulting in easier vaporization at lower temperatures. Ionic compounds, due to their strong electrostatic forces, have significantly lower volatility.
Examples: A Practical Comparison
Let's consider some practical examples to illustrate these differences:
-
Sodium Chloride (NaCl): A typical ionic compound with a high melting point (801 °C), high boiling point (1413 °C), brittle, soluble in water, and conducts electricity when molten or dissolved in water.
-
Diamond (C): A covalent network solid, exhibiting exceptional hardness, high melting point, and poor conductivity. While it is a covalent compound, its unique structure makes it exceptional.
-
Sucrose (C₁₂H₂₂O₁₁): A molecular compound with a relatively low melting point (186 °C), soluble in water, and a poor conductor of electricity.
-
Water (H₂O): A polar molecular compound, having unique properties due to strong hydrogen bonding. It has a relatively low melting point (0 °C) and boiling point (100 °C), is a good solvent for polar substances, and does not conduct electricity unless impurities are present, which then form ions.
Conclusion: Understanding the Distinctions
The differences between solid ionic and solid molecular compounds are profound and stem from the fundamental distinctions in their chemical bonding and crystal structures. These differences manifest in their vastly different physical properties, impacting their applications in various fields. Understanding these differences is critical for choosing the right materials for specific purposes. Whether designing new materials, developing pharmaceuticals, or understanding geological processes, a grasp of the unique characteristics of these compound types is essential. This knowledge empowers scientists and engineers to harness the properties of these materials to create innovative solutions and technologies.
Latest Posts
Latest Posts
-
Which Is The Electron Configuration For Lithium
Mar 23, 2025
-
Integration By Parts Examples With Solutions
Mar 23, 2025
-
Where In The Cell Does Fermentation Occur
Mar 23, 2025
-
What Is The Acceptable Macronutrient Distribution Range For Carbohydrates
Mar 23, 2025
-
How To Show Vectors Are Linearly Independent
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Compared With Solid Ionic Compounds Solid Molecular Compounds Generally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
