Which Is The Electron Configuration For Lithium
Muz Play
Mar 23, 2025 · 7 min read
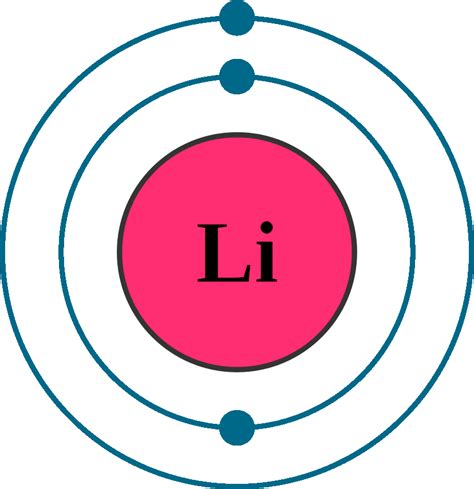
Table of Contents
Which is the Electron Configuration for Lithium? A Deep Dive into Atomic Structure
Lithium, the lightest of the alkali metals, holds a significant place in chemistry and physics. Understanding its electron configuration is crucial for grasping its chemical behavior and properties. This article delves deep into the electron configuration of lithium, exploring the underlying principles of atomic structure and explaining its significance in various contexts. We'll also touch upon related concepts like quantum numbers, orbital shapes, and the periodic table's organization.
Understanding Electron Configuration
The electron configuration of an atom describes how electrons are distributed among the various energy levels and sublevels within the atom. It follows specific rules dictated by quantum mechanics, helping us predict an element's reactivity and bonding behavior. The configuration is expressed using a notation that specifies the principal energy level (n), the sublevel (s, p, d, or f), and the number of electrons in each sublevel.
For instance, the electron configuration of an atom is written as 1s²2s²2p⁶3s¹ if it has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, and one electron in the 3s orbital.
Lithium's Atomic Structure: A Simple Yet Powerful Atom
Lithium (Li), with an atomic number of 3, possesses three protons in its nucleus and, in its neutral state, three electrons orbiting the nucleus. These electrons occupy specific energy levels and orbitals, determined by the principles of quantum mechanics.
The Significance of Atomic Number
The atomic number, 3 in lithium's case, directly dictates the number of electrons in a neutral atom. Each electron occupies an orbital, which is a region of space where there's a high probability of finding the electron.
Determining Lithium's Electron Configuration: A Step-by-Step Approach
To determine lithium's electron configuration, we follow the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
1. The Aufbau Principle: Filling Orbitals in Order of Increasing Energy
The Aufbau principle states that electrons fill the lowest energy levels first. The order of filling is:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p…
2. Hund's Rule: Maximizing Unpaired Electrons
Hund's rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion.
3. The Pauli Exclusion Principle: A Maximum of Two Electrons per Orbital
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers (principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number). This means each orbital can hold a maximum of two electrons, and these electrons must have opposite spins.
Applying the Rules to Lithium
Lithium has three electrons. Following the Aufbau principle, we fill the orbitals in order of increasing energy:
- 1s orbital: The lowest energy level, the 1s orbital, can hold up to two electrons. Lithium's first two electrons fill this orbital. We represent this as 1s².
- 2s orbital: The next lowest energy level is the 2s orbital, which can also hold up to two electrons. Lithium's third electron occupies this orbital. We represent this as 2s¹.
Therefore, the complete electron configuration for lithium is 1s²2s¹.
Visualizing Lithium's Electron Configuration: Orbital Diagrams
Orbital diagrams provide a visual representation of electron configuration. Each orbital is represented by a box, and electrons are represented by arrows. Arrows pointing up and down represent electrons with opposite spins.
For lithium, the orbital diagram would look like this:
1s: ↑↓ 2s: ↑
This clearly shows that the 1s orbital is filled with two electrons, while the 2s orbital contains only one electron.
The Significance of Lithium's Electron Configuration
Lithium's 1s²2s¹ configuration is crucial in understanding its chemical properties and behavior.
Reactivity and Valence Electrons
The outermost electron in the 2s orbital is the valence electron. Valence electrons are responsible for chemical bonding. Lithium readily loses this valence electron to achieve a stable, noble gas configuration (like helium, 1s²), making it highly reactive. This explains its propensity to form ionic compounds by losing an electron to become a positively charged ion (Li⁺).
Bonding Characteristics
The ease with which lithium loses its valence electron explains its low ionization energy. This means it takes relatively little energy to remove the electron, resulting in the formation of ionic bonds with electronegative elements. Lithium forms ionic compounds with non-metals, such as lithium chloride (LiCl) and lithium oxide (Li₂O).
Spectral Lines and Quantum Jumps
The electron configuration also dictates the spectral lines observed when lithium atoms are excited. When an electron absorbs energy, it can jump to a higher energy level. When it falls back to a lower energy level, it emits a photon of light with a specific wavelength, corresponding to the energy difference between the levels. The 1s²2s¹ configuration determines the possible energy transitions and the resulting spectral lines.
Comparing Lithium's Electron Configuration to Other Elements
Comparing lithium's electron configuration to other elements in the same group (alkali metals) and period (second period) reveals trends in chemical properties.
Alkali Metals: Similar Configurations
Other alkali metals (sodium, potassium, rubidium, cesium, and francium) share a similar electron configuration pattern, with one valence electron in the outermost s orbital. This similarity explains the shared chemical properties within the group, like high reactivity and the formation of +1 ions.
Second Period Elements: Different Configurations, Different Properties
Elements in the second period (lithium to neon) exhibit diverse properties due to their varying electron configurations. For example, beryllium (1s²2s²) has two valence electrons and forms covalent bonds more readily than lithium. The subsequent elements (boron, carbon, nitrogen, oxygen, fluorine, and neon) show variations in bonding characteristics and electronegativity, dictated by the electron configurations of their valence shells.
The Role of Quantum Numbers in Defining Lithium's Electron Configuration
Quantum numbers are essential in describing the properties of electrons in an atom and, therefore, its electron configuration.
-
Principal Quantum Number (n): Represents the energy level (shell) and is a positive integer (1, 2, 3...). For lithium's electrons, n=1 for the 1s electrons and n=2 for the 2s electron.
-
Azimuthal Quantum Number (l): Represents the sublevel (orbital shape) and can range from 0 to n-1. For s orbitals, l=0; for p orbitals, l=1; for d orbitals, l=2; and for f orbitals, l=3. Lithium's 1s and 2s electrons have l=0.
-
Magnetic Quantum Number (ml): Represents the orientation of the orbital in space and ranges from -l to +l. For s orbitals, ml=0. Lithium's electrons in both the 1s and 2s orbitals have ml=0.
-
Spin Quantum Number (ms): Represents the electron's intrinsic angular momentum (spin) and can be +1/2 or -1/2. Lithium's two 1s electrons have opposite spins (+1/2 and -1/2), while the 2s electron has either +1/2 or -1/2 spin.
Advanced Concepts and Applications
Understanding lithium's electron configuration opens the door to numerous advanced concepts:
-
Photoelectron Spectroscopy: This technique can experimentally determine the ionization energies of electrons in an atom, providing direct evidence for the energy levels and electron configuration.
-
Molecular Orbital Theory: This theory explains the bonding in molecules by considering the combination of atomic orbitals to form molecular orbitals. Understanding lithium's atomic orbitals is fundamental to applying molecular orbital theory to lithium-containing molecules.
-
Quantum Chemistry Calculations: Sophisticated computational methods, based on quantum mechanics, can predict various properties of lithium and its compounds, such as bond lengths, bond energies, and reactivity, by considering the atom's electron configuration and its interaction with other atoms.
Conclusion
The electron configuration of lithium, 1s²2s¹, is a cornerstone for understanding its chemical behavior and reactivity. This simple yet powerful configuration highlights the fundamental principles of atomic structure and the importance of quantum mechanics in explaining the properties of matter. By grasping this configuration, we gain insights into lithium's role in various chemical reactions, its bonding characteristics, its spectral lines, and its place within the periodic table. Furthermore, it serves as a stepping stone to exploring advanced concepts in chemistry and physics.
Latest Posts
Latest Posts
-
A Case Of Cystic Fibrosis Answer Key
Mar 24, 2025
-
How Do Plants Respond To Their Environment
Mar 24, 2025
-
Poe Is Considered The Father Of
Mar 24, 2025
-
The Rows In The Periodic Table Are Called
Mar 24, 2025
-
How To Determine Profit Maximizing Price
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Which Is The Electron Configuration For Lithium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
