The Rows In The Periodic Table Are Called
Muz Play
Mar 24, 2025 · 6 min read
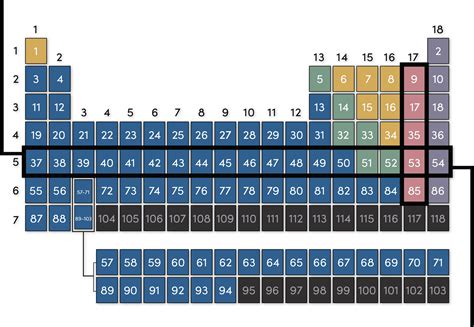
Table of Contents
The Rows in the Periodic Table are Called Periods: A Deep Dive into Atomic Structure and Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its organization is crucial to grasping the relationships between elements and predicting their behavior. While many are familiar with the table's columns, called groups or families, fewer understand the significance of its rows, which are formally known as periods. This article delves deep into the concept of periods, exploring their connection to electron shells, atomic size, ionization energy, and electronegativity. We’ll also examine how the periodic properties of elements change across a period, and the impact of this on chemical reactivity.
What are Periods in the Periodic Table?
The rows in the periodic table are called periods. Each period represents a principal energy level or shell in an atom. As we move across a period from left to right, we add one proton and one electron to the atom. This systematic addition of electrons fills the orbitals within a given energy level. Crucially, all elements within a given period share the same highest occupied principal quantum number (n). This quantum number defines the energy level of the outermost electrons, significantly impacting the element's chemical behavior.
The Significance of Principal Quantum Number (n)
The principal quantum number (n) is a fundamental concept in quantum mechanics that describes the energy level of an electron. It can take on positive integer values (1, 2, 3, etc.). The higher the value of n, the higher the energy level and the further the electron is from the nucleus. Elements in the same period share the same value of n for their outermost electrons. For example, all elements in period 1 (hydrogen and helium) have their outermost electrons in the n=1 energy level, while all elements in period 2 (lithium to neon) have their outermost electrons in the n=2 energy level.
Periodicity of Properties Across a Period
The periodic table derives its name from the periodic recurrence of similar properties in elements as you move across and down the table. These periodic properties are closely tied to the electron configuration of the atoms. Let's analyze how these properties vary across a period:
1. Atomic Radius: A Gradual Decrease
Atomic radius refers to the distance from the nucleus to the outermost electron. As we move from left to right across a period, the atomic radius generally decreases. This is because the number of protons in the nucleus increases, resulting in a stronger positive charge that pulls the electrons closer. While additional electrons are added, they are entering the same principal energy level, and the increased nuclear charge outweighs the electron-electron repulsion, leading to a smaller atomic size.
2. Ionization Energy: A General Increase
Ionization energy is the energy required to remove an electron from a neutral atom. As we move across a period, the ionization energy generally increases. This is a direct consequence of the increasing nuclear charge. The stronger attraction between the nucleus and the electrons makes it increasingly difficult to remove an electron. Exceptions can occur due to electron shielding and electron-electron repulsion, particularly when moving from a half-filled or fully-filled subshell to the next.
3. Electronegativity: A Rising Trend
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Similar to ionization energy, electronegativity generally increases across a period. The increased nuclear charge makes the atom more capable of attracting electrons from other atoms. The noble gases (Group 18) are exceptions, having very low electronegativity due to their stable electron configurations.
4. Metallic Character: A Transition from Metal to Nonmetal
Metallic character refers to the properties associated with metals, such as conductivity, malleability, and ductility. Across a period, metallic character generally decreases. Elements on the left side of a period tend to be metals, while elements on the right side tend to be nonmetals. This trend is a reflection of the increasing ionization energy and electronegativity. Metals readily lose electrons to form positive ions, while nonmetals tend to gain electrons to form negative ions.
5. Reactivity: A Complex Relationship
The reactivity of elements is a complex property influenced by several factors, including ionization energy, electronegativity, and electron configuration. In general, the reactivity of metals (on the left side of a period) decreases as we move from left to right. However, the reactivity of nonmetals (on the right side) increases as we move from left to right, culminating in highly reactive halogens. The noble gases are largely unreactive due to their full electron shells.
Periods and Electron Configurations
Each period corresponds to the filling of a principal energy level or shell. Let's break this down by period:
-
Period 1 (n=1): Contains only two elements, hydrogen and helium, which fill the 1s subshell.
-
Period 2 (n=2): Contains eight elements (lithium to neon), filling the 2s and 2p subshells.
-
Period 3 (n=3): Also contains eight elements (sodium to argon), filling the 3s and 3p subshells.
-
Period 4 (n=4): Contains eighteen elements, filling the 4s, 3d, and 4p subshells. The introduction of the d subshell leads to the transition metals.
-
Period 5 (n=5): Similar to period 4, it contains eighteen elements filling the 5s, 4d, and 5p subshells.
-
Period 6 (n=6): Contains thirty-two elements due to the addition of the 4f subshell (lanthanides) along with 6s, 5d, and 6p subshells.
-
Period 7 (n=7): The longest period, it also contains thirty-two elements with similar subshell filling as period 6, along with the actinides filling the 5f subshell.
The filling of subshells within a period dictates the electron configuration and ultimately, the chemical properties of the elements. The transition metals (d-block) and inner transition metals (f-block) represent nuances within this filling pattern, resulting in variations in the trends described earlier.
Exceptions and Irregularities
While the trends described above are generally followed, there are exceptions and irregularities. These exceptions often arise due to:
-
Electron shielding: Inner electrons partially shield the outer electrons from the full effect of the nuclear charge, leading to variations in effective nuclear charge.
-
Electron-electron repulsion: Repulsion between electrons in the same subshell can influence atomic properties.
-
Half-filled and fully-filled subshells: Atoms with half-filled or fully-filled subshells have added stability, influencing their ionization energy and other properties.
Understanding these exceptions requires a deeper dive into quantum mechanics and atomic structure.
The Importance of Understanding Periods
Understanding periods in the periodic table is critical for:
-
Predicting chemical properties: Knowing an element's period allows us to predict its atomic radius, ionization energy, electronegativity, and metallic character, providing insights into its reactivity and bonding behavior.
-
Understanding chemical trends: The periodic repetition of properties allows us to establish patterns and predict the properties of undiscovered elements.
-
Interpreting chemical reactions: Understanding the electronic structure of atoms, related to their position within a period, is crucial to interpreting the mechanisms of chemical reactions.
-
Developing new materials: Knowledge of periodic trends is invaluable in material science for designing and synthesizing materials with desired properties.
In conclusion, the rows in the periodic table, known as periods, are fundamental to the organization and understanding of the periodic table. They reflect the systematic addition of electrons to the principal energy levels of atoms, resulting in predictable trends in atomic properties. By understanding the relationship between periods, electron configurations, and atomic properties, we gain a powerful tool for predicting and interpreting chemical behavior. While exceptions and irregularities exist, the overall trends across periods offer a valuable framework for comprehending the diversity and interconnectedness of the elements.
Latest Posts
Latest Posts
-
Plot A Normal Distribution In R
Mar 26, 2025
-
Place The Steps Of Eukaryotic Transcription In Order Of Occurrence
Mar 26, 2025
-
Where Is The Mass Of The Atom Located
Mar 26, 2025
-
Which Organ Is An Accessory Organ Of Digestion
Mar 26, 2025
-
What Is The Difference Between Gene Mutations And Chromosomal Mutations
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about The Rows In The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
