Where Is The Mass Of The Atom Located
Muz Play
Mar 26, 2025 · 6 min read
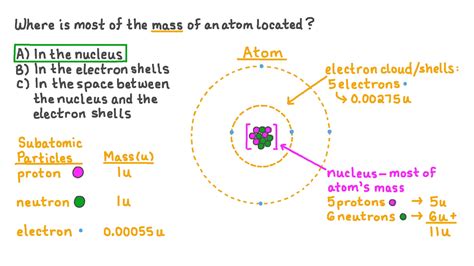
Table of Contents
- Where Is The Mass Of The Atom Located
- Table of Contents
- Where is the Mass of the Atom Located? Unpacking the Atomic Nucleus
- Delving into Atomic Structure: A Brief Overview
- The Mass of Subatomic Particles: A Crucial Distinction
- The Nucleus: The Mass Hub of the Atom
- The Strong Nuclear Force: The Glue Holding the Nucleus Together
- Isotopes and Atomic Mass: Variations on a Theme
- Beyond Atomic Mass: Introducing Atomic Weight
- The Implications of Nuclear Mass: Applications and Discoveries
- Beyond the Basics: Subatomic Structure and Quantum Mechanics
- Conclusion: A Tiny Core, a Huge Impact
- Latest Posts
- Latest Posts
- Related Post
Where is the Mass of the Atom Located? Unpacking the Atomic Nucleus
The seemingly simple question, "Where is the mass of the atom located?" unlocks a fascinating journey into the heart of matter, revealing the intricate structure of atoms and the fundamental forces that govern the universe. While the atom as a whole possesses mass, the bulk of this mass isn't uniformly distributed. It's concentrated in a tiny, dense region at the atom's core: the nucleus.
Delving into Atomic Structure: A Brief Overview
Before we pinpoint the location of atomic mass, let's establish a foundational understanding of atomic structure. Atoms, the fundamental building blocks of matter, are composed of three primary subatomic particles:
- Protons: Positively charged particles residing within the nucleus.
- Neutrons: Neutral particles (no charge) also found within the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The nucleus, therefore, is the central core of the atom, containing both protons and neutrons. The electrons, significantly lighter than protons and neutrons, occupy the space surrounding the nucleus, forming a sort of electron cloud. This cloud isn't a sharply defined boundary but rather a region of probability where electrons are most likely to be found.
The Mass of Subatomic Particles: A Crucial Distinction
The mass of an atom is primarily determined by the combined mass of its protons and neutrons. Electrons contribute a negligible amount to the overall mass. To illustrate:
- Proton Mass: Approximately 1.67 x 10^-27 kg
- Neutron Mass: Approximately 1.67 x 10^-27 kg
- Electron Mass: Approximately 9.11 x 10^-31 kg
Notice the significant difference in mass between protons/neutrons and electrons. The electron's mass is roughly 1/1836 the mass of a proton or neutron. This stark contrast highlights the dominance of the nucleus in determining the atom's overall mass.
The Nucleus: The Mass Hub of the Atom
The incredibly small size of the nucleus compared to the overall atom is astonishing. Imagine a stadium representing an atom; the nucleus would be a tiny marble at the center. Despite its minuscule size, the nucleus packs almost all of the atom's mass. This extraordinary density is a consequence of the strong nuclear force.
The Strong Nuclear Force: The Glue Holding the Nucleus Together
The strong nuclear force is one of the four fundamental forces in nature, and it's responsible for binding protons and neutrons together within the nucleus. This force is incredibly powerful at short distances, overcoming the electrostatic repulsion between the positively charged protons. Without the strong nuclear force, the nucleus would instantly fly apart.
The strength of the strong nuclear force is crucial in determining the stability of the nucleus and, consequently, the atom's overall stability. The ratio of protons to neutrons in the nucleus plays a significant role in nuclear stability, influencing an element's radioactivity.
Isotopes and Atomic Mass: Variations on a Theme
While the number of protons defines an element (e.g., all atoms with one proton are hydrogen), the number of neutrons can vary. These variations are called isotopes. Isotopes of the same element have the same number of protons but different numbers of neutrons. This difference in neutron number affects the atom's mass.
For instance, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are carbon isotopes, but ¹⁴C is heavier due to the extra two neutrons. The atomic mass number (often rounded to the nearest whole number) represents the total number of protons and neutrons in an atom's nucleus. This mass number is a convenient way to represent the mass of an atom, acknowledging the near-negligible contribution of electrons.
Beyond Atomic Mass: Introducing Atomic Weight
The term "atomic weight" (or relative atomic mass) often appears in chemistry and physics. It's important to differentiate it from atomic mass. Atomic weight is the weighted average of the masses of all naturally occurring isotopes of an element. This weighted average reflects the abundance of each isotope in nature. Because isotopes have different masses and abundances, the atomic weight differs slightly from the mass number of the most abundant isotope.
The Implications of Nuclear Mass: Applications and Discoveries
Understanding the location and significance of the atomic nucleus's mass has far-reaching implications across various scientific disciplines:
-
Nuclear Physics: This field investigates the properties and behavior of atomic nuclei, leading to breakthroughs in nuclear energy, nuclear medicine (radioactive isotopes for diagnosis and treatment), and nuclear weapons technology. The knowledge of nuclear mass is fundamental in these applications.
-
Nuclear Chemistry: This area explores the chemical changes that result from nuclear reactions. Radioactive isotopes, with their specific mass and decay properties, are vital tools in many chemical analyses and dating techniques (like carbon dating).
-
Material Science: The properties of materials are intimately linked to the atomic structure and interactions within their constituent atoms. Understanding the mass distribution within atoms is critical in developing new materials with tailored properties, for instance, stronger alloys or more efficient semiconductors.
-
Astrophysics: Nuclear processes are the driving force behind the energy production in stars. The fusion of atomic nuclei, which involves changes in nuclear mass, fuels the stars and produces heavier elements. Understanding these processes is essential to comprehending stellar evolution and the origin of elements in the universe.
Beyond the Basics: Subatomic Structure and Quantum Mechanics
While the simple model of protons, neutrons, and electrons suffices for many purposes, a deeper dive into subatomic structure reveals a more complex reality. Protons and neutrons themselves are composed of even smaller particles called quarks. The interaction between quarks, mediated by gluons, is described by the theory of Quantum Chromodynamics (QCD), a complex part of the Standard Model of particle physics.
Quantum mechanics, the framework governing the behavior of particles at the atomic and subatomic levels, provides a probabilistic description of electron location. Electrons don't follow well-defined orbits like planets around a star. Instead, their behavior is governed by wave functions that describe the probability of finding an electron at a particular location within the electron cloud.
Conclusion: A Tiny Core, a Huge Impact
The answer to "Where is the mass of the atom located?" is unequivocally: the nucleus. This tiny, dense region at the atom's center contains almost all of an atom's mass, primarily due to the protons and neutrons it houses. The strong nuclear force binds these particles together, creating a remarkably stable core that dictates the atom's properties and plays a crucial role in shaping the world around us. Understanding the nucleus's mass and its implications has been pivotal to countless scientific advancements and technological innovations. From harnessing nuclear energy to developing new materials and understanding the cosmos, the journey into the heart of the atom continues to reveal the universe's profound complexities and beauty.
Latest Posts
Latest Posts
-
What Are The Elements In Group 18 Called
Mar 29, 2025
-
How Do You Make A Standard Curve
Mar 29, 2025
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
-
Equation Of Tangent Line Implicit Differentiation
Mar 29, 2025
-
According To The Bronsted Lowry Definition
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Where Is The Mass Of The Atom Located . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
