According To The Bronsted Lowry Definition
Muz Play
Mar 29, 2025 · 6 min read
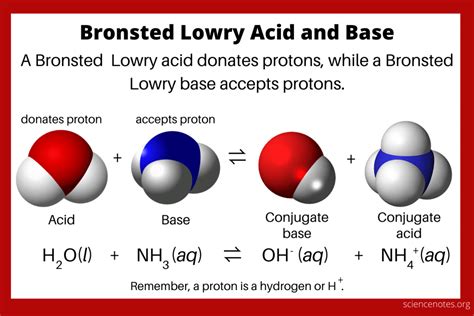
Table of Contents
According to the Brønsted-Lowry Definition: A Deep Dive into Acid-Base Chemistry
The Brønsted-Lowry definition of acids and bases revolutionized our understanding of acid-base chemistry, moving beyond the limitations of the Arrhenius definition. This comprehensive guide delves into the core principles of the Brønsted-Lowry theory, exploring its strengths, applications, and nuances. We'll examine conjugate acid-base pairs, amphoteric substances, and the implications for various chemical reactions. By the end, you'll possess a robust understanding of this foundational concept in chemistry.
Understanding the Brønsted-Lowry Definition
Unlike the Arrhenius definition, which restricts acids to substances that produce H⁺ ions (protons) in aqueous solution and bases to those that produce OH⁻ ions (hydroxide ions), the Brønsted-Lowry definition provides a broader perspective. It defines:
- Acid: A Brønsted-Lowry acid is any species that can donate a proton (H⁺) to another species.
- Base: A Brønsted-Lowry base is any species that can accept a proton (H⁺) from another species.
This expanded definition allows for acid-base reactions to occur in non-aqueous solutions, significantly broadening the scope of acid-base chemistry. The key is the transfer of a proton, not the presence of water.
Key Differences from the Arrhenius Definition
The Arrhenius definition, while useful for understanding some acid-base reactions, is limited in several ways:
- Solvent Dependency: The Arrhenius definition is heavily reliant on aqueous solutions. Reactions in non-aqueous solvents aren't considered acid-base reactions according to this definition.
- Limited Scope: It only accounts for acids producing H⁺ and bases producing OH⁻. Many substances exhibiting acidic or basic properties don't fit this framework.
The Brønsted-Lowry definition overcomes these limitations by focusing on proton transfer, making it a more versatile and encompassing theory.
Conjugate Acid-Base Pairs
A crucial concept within the Brønsted-Lowry framework is the conjugate acid-base pair. When an acid donates a proton, it forms its conjugate base, which is the species remaining after the proton is lost. Conversely, when a base accepts a proton, it forms its conjugate acid.
Let's illustrate this with an example: the reaction between hydrochloric acid (HCl) and water (H₂O).
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
In this reaction:
- HCl acts as the acid, donating a proton to water.
- H₂O acts as the base, accepting a proton from HCl.
- Cl⁻ is the conjugate base of HCl.
- H₃O⁺ (hydronium ion) is the conjugate acid of H₂O.
Notice that the conjugate base (Cl⁻) has one less proton than its corresponding acid (HCl), and the conjugate acid (H₃O⁺) has one more proton than its corresponding base (H₂O). This proton transfer is the defining characteristic of a Brønsted-Lowry acid-base reaction.
Identifying Conjugate Pairs
Identifying conjugate acid-base pairs is a fundamental skill in Brønsted-Lowry acid-base chemistry. To do so, look for the species that differ by only one proton (H⁺). The species with the extra proton is the acid, and the species with one less proton is the base.
Amphoteric Substances: Acting as Both Acid and Base
An amphoteric substance is a species that can act as both a Brønsted-Lowry acid and a Brønsted-Lowry base, depending on the reaction conditions. Water is a classic example of an amphoteric substance.
Consider the following reactions:
- Water as an acid: H₂O(l) + NH₃(aq) ⇌ OH⁻(aq) + NH₄⁺(aq) (Water donates a proton to ammonia)
- Water as a base: H₂O(l) + HCl(aq) ⇌ H₃O⁺(aq) + Cl⁻(aq) (Water accepts a proton from hydrochloric acid)
In the first reaction, water acts as an acid, donating a proton to ammonia (NH₃). In the second reaction, water acts as a base, accepting a proton from hydrochloric acid (HCl). This dual capability highlights the amphoteric nature of water.
Other amphoteric substances include bicarbonate ion (HCO₃⁻), hydrogen sulfate ion (HSO₄⁻), and many metal hydroxides. The ability to act as both an acid and a base is determined by the substance's chemical structure and its ability to either donate or accept a proton.
Applications of the Brønsted-Lowry Definition
The Brønsted-Lowry definition has profound implications across various chemical fields, including:
- Understanding Biochemical Processes: Many biochemical reactions involve proton transfer, such as enzyme catalysis and protein folding. The Brønsted-Lowry definition provides the framework for understanding these processes.
- Environmental Chemistry: Acid rain, a significant environmental problem, is readily explained using the Brønsted-Lowry definition. Acidic gases like sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) react with water in the atmosphere to form acids.
- Industrial Processes: Many industrial processes, such as the production of fertilizers and pharmaceuticals, rely on acid-base reactions. The Brønsted-Lowry definition helps optimize these processes.
- Analytical Chemistry: Titrations, a common analytical technique, utilize acid-base reactions to determine the concentration of unknown solutions. The Brønsted-Lowry definition provides the theoretical basis for these analyses.
Strengths and Limitations of the Brønsted-Lowry Theory
While the Brønsted-Lowry definition significantly expands on the Arrhenius definition, it does have some limitations:
- Proton Transfer Focus: The theory focuses solely on proton transfer. Reactions involving electron transfer or other types of interactions aren't considered acid-base reactions under this definition.
- Solvent Effects: While less dependent on the solvent than the Arrhenius definition, the Brønsted-Lowry definition still doesn't fully account for the role of the solvent in acid-base reactions.
Despite these limitations, the Brønsted-Lowry definition remains a cornerstone of acid-base chemistry due to its comprehensive scope and applicability to a wide range of reactions.
Beyond Protons: Lewis Acids and Bases
Even the Brønsted-Lowry definition doesn't encompass all acid-base reactions. The Lewis definition provides an even broader perspective, defining acids and bases based on electron pairs rather than proton transfer.
- Lewis Acid: A Lewis acid is an electron-pair acceptor.
- Lewis Base: A Lewis base is an electron-pair donor.
Many substances that are not Brønsted-Lowry acids or bases can be classified as Lewis acids or bases. For example, BF₃ (boron trifluoride) acts as a Lewis acid by accepting an electron pair from a Lewis base like ammonia (NH₃). While this reaction doesn't involve proton transfer, it's still considered an acid-base reaction according to the Lewis definition.
Conclusion: The Enduring Significance of Brønsted-Lowry Theory
The Brønsted-Lowry definition is a pivotal advancement in acid-base chemistry. Its focus on proton transfer provides a more comprehensive and versatile framework than the Arrhenius definition, enabling a deeper understanding of a vast array of chemical reactions. While limitations exist, its strengths far outweigh its weaknesses, making it a crucial concept in chemistry education and research. Understanding conjugate acid-base pairs, amphoteric substances, and the limitations of the theory are essential for a complete grasp of this fundamental aspect of chemistry. The ability to apply this understanding to practical scenarios in various fields further emphasizes the enduring significance of the Brønsted-Lowry definition. Moving beyond this foundational understanding naturally leads to a deeper appreciation of the broader Lewis definition, further enriching one’s comprehension of acid-base interactions.
Latest Posts
Latest Posts
-
How To Find C In Sinusoidal Function
Apr 01, 2025
-
How To Solve System Of Equations With Three Variables
Apr 01, 2025
-
Lewis Dot Diagram For 2 Individual Ions For Na
Apr 01, 2025
-
Three Stages Of The Perception Process
Apr 01, 2025
-
Difference Between Solution Suspension And Colloid
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about According To The Bronsted Lowry Definition . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
