Compounds That React With Acids To Form Salts
Muz Play
Apr 07, 2025 · 6 min read
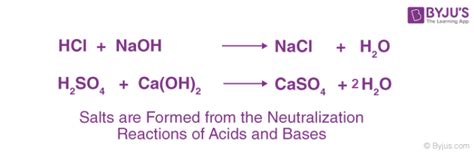
Table of Contents
Compounds That React with Acids to Form Salts
Many compounds react with acids to produce salts. Understanding these reactions is crucial in chemistry, impacting various fields from industrial processes to biological functions. This comprehensive guide delves into the diverse classes of compounds that undergo these reactions, the mechanisms involved, and the properties of the resulting salts.
Bases and Their Reactions with Acids
The most common compounds reacting with acids to form salts are bases. Bases are substances that can accept protons (H⁺ ions) from acids. This reaction, known as neutralization, is a fundamental concept in acid-base chemistry.
Strong Bases and Weak Bases
The strength of a base dictates the extent of its reaction with an acid. Strong bases, like sodium hydroxide (NaOH) and potassium hydroxide (KOH), completely dissociate in water, releasing hydroxide ions (OH⁻) that readily react with protons from acids. The reaction is essentially complete, leaving minimal unreacted base.
Example: The reaction between sodium hydroxide and hydrochloric acid (HCl) produces sodium chloride (NaCl) and water:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
Weak bases, such as ammonia (NH₃) and amines, only partially dissociate in water, resulting in a lower concentration of hydroxide ions. Their reaction with acids is an equilibrium process, meaning some unreacted base remains.
Example: The reaction between ammonia and hydrochloric acid produces ammonium chloride (NH₄Cl):
NH₃(aq) + HCl(aq) ⇌ NH₄Cl(aq)
Metal Oxides and Hydroxides
Metal oxides and hydroxides are quintessential examples of bases. Metal oxides react with acids to form salts and water.
Example: The reaction between copper(II) oxide (CuO) and sulfuric acid (H₂SO₄) produces copper(II) sulfate (CuSO₄) and water:
CuO(s) + H₂SO₄(aq) → CuSO₄(aq) + H₂O(l)
Similarly, metal hydroxides react with acids to yield salts and water. This is a classic neutralization reaction.
Example: The reaction of aluminum hydroxide (Al(OH)₃) with nitric acid (HNO₃) forms aluminum nitrate (Al(NO₃)₃) and water:
Al(OH)₃(s) + 3HNO₃(aq) → Al(NO₃)₃(aq) + 3H₂O(l)
Metal Carbonates and Bicarbonates
Metal carbonates (CO₃²⁻) and bicarbonates (HCO₃⁻) also react with acids. These reactions produce salts, carbon dioxide gas (CO₂), and water. The release of carbon dioxide is a characteristic feature of these reactions.
Reaction with Carbonates
The reaction between a metal carbonate and an acid is effervescent due to the release of carbon dioxide.
Example: The reaction of calcium carbonate (CaCO₃) with hydrochloric acid (HCl) yields calcium chloride (CaCl₂), carbon dioxide, and water:
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + CO₂(g) + H₂O(l)
This reaction is frequently used to test for the presence of carbonates. The effervescence, coupled with the characteristic properties of carbon dioxide, confirms the presence of the carbonate ion.
Reaction with Bicarbonates
Bicarbonates also react with acids, producing similar products. However, the reaction is generally less vigorous than with carbonates.
Example: Sodium bicarbonate (NaHCO₃) reacting with acetic acid (CH₃COOH) forms sodium acetate (CH₃COONa), carbon dioxide, and water:
NaHCO₃(s) + CH₃COOH(aq) → CH₃COONa(aq) + CO₂(g) + H₂O(l)
This reaction is often exploited in baking, where the carbon dioxide produced helps to leaven the dough.
Metal Sulfides
Metal sulfides (S²⁻) react with acids to form salts and hydrogen sulfide gas (H₂S). Hydrogen sulfide is a highly toxic and foul-smelling gas. Therefore, these reactions should be performed under a well-ventilated area or using appropriate safety measures.
Example: The reaction between iron(II) sulfide (FeS) and hydrochloric acid (HCl) produces iron(II) chloride (FeCl₂), and hydrogen sulfide:
FeS(s) + 2HCl(aq) → FeCl₂(aq) + H₂S(g)
The characteristic rotten egg smell of hydrogen sulfide readily identifies this reaction.
Ammonia and Amines
Ammonia (NH₃) and amines (organic compounds containing an amino group, -NH₂) act as weak bases. They react with acids to form ammonium salts and substituted ammonium salts, respectively.
Reaction of Ammonia with Acids
Ammonia reacts with acids to form ammonium salts. These salts are generally ionic compounds that are soluble in water.
Example: The reaction between ammonia and sulfuric acid produces ammonium sulfate:
2NH₃(aq) + H₂SO₄(aq) → (NH₄)₂SO₄(aq)
Reaction of Amines with Acids
Amines react similarly with acids, forming substituted ammonium salts.
Example: The reaction between methylamine (CH₃NH₂) and hydrochloric acid produces methylammonium chloride:
CH₃NH₂(aq) + HCl(aq) → CH₃NH₃Cl(aq)
These reactions are important in organic chemistry and biochemistry, playing a role in various synthesis and biological processes.
Other Compounds Forming Salts with Acids
While bases are the most common type of compounds that react with acids to form salts, other compounds can also participate in such reactions under specific conditions. These include certain metal oxides, sulfides, and even some organic compounds exhibiting basic properties.
Amphoteric Oxides and Hydroxides
Amphoteric oxides and hydroxides can react with both acids and bases. This unique property allows them to form salts with acids.
Example: Aluminum hydroxide (Al(OH)₃) reacts with hydrochloric acid (HCl) to form aluminum chloride (AlCl₃):
Al(OH)₃(s) + 3HCl(aq) → AlCl₃(aq) + 3H₂O(l)
Organic Compounds with Basic Properties
Some organic compounds, containing functional groups like amines or carboxylates, possess basic properties and can react with acids to form salts.
Example: A reaction between a carboxylic acid and a strong base, like NaOH, can produce a carboxylate salt and water. These carboxylate salts can further react with acids.
Properties of Salts Formed
The salts produced from the reaction of acids with various compounds exhibit a range of properties depending on the constituent ions. Some common properties include:
- Ionic Nature: Most salts are ionic compounds, consisting of positively charged cations and negatively charged anions.
- Solubility: The solubility of salts varies greatly depending on the cation and anion. Some salts are highly soluble in water (e.g., NaCl), while others are insoluble (e.g., AgCl).
- Electrical Conductivity: Dissolved salts in water conduct electricity because of the presence of mobile ions.
- pH: The pH of a salt solution depends on the strength of the acid and base from which it is formed. Salts derived from a strong acid and a strong base have a neutral pH. Salts from a strong acid and a weak base have an acidic pH, while salts from a weak acid and a strong base have an alkaline pH.
- Crystal Structure: Salts typically exhibit a crystalline structure, with the ions arranged in a regular three-dimensional lattice.
Applications of Salt Formation Reactions
Reactions involving acid-base neutralization and salt formation have numerous applications across diverse fields:
- Industrial Processes: Salt formation reactions are crucial in many industrial processes, such as the production of fertilizers, pigments, and pharmaceuticals.
- Analytical Chemistry: Neutralization titrations are used extensively in analytical chemistry to determine the concentration of acids and bases.
- Environmental Remediation: Acid-base reactions are employed in environmental remediation to neutralize acidic or alkaline spills.
- Biological Systems: Acid-base reactions and salt formation play vital roles in maintaining the pH balance in biological systems. Buffer systems, which resist changes in pH, are based on the equilibrium between weak acids and their conjugate bases.
Understanding the reactions of compounds with acids to form salts is essential for a thorough grasp of chemistry. This knowledge finds applications across various scientific disciplines and industries, highlighting the importance of this fundamental chemical process. The specific properties of the resulting salt depend on the reactants involved, paving the way for the creation of tailored materials with specific functionalities. Furthermore, the principles involved extend far beyond the laboratory, shaping crucial processes in the environment and in biological systems.
Latest Posts
Latest Posts
-
Is Sodium A Substance Or Mixture
Apr 08, 2025
-
One Of The Functions Of Nonverbal Communication Is Forming
Apr 08, 2025
-
Is P Value The Same As Critical Value
Apr 08, 2025
-
Has Properties Of Both Metals And Nonmetals
Apr 08, 2025
-
Internal And External Users Of Accounting
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Compounds That React With Acids To Form Salts . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.