Dialysis Can Be Used To Separate Solutions From Colloids.
Muz Play
Mar 16, 2025 · 5 min read
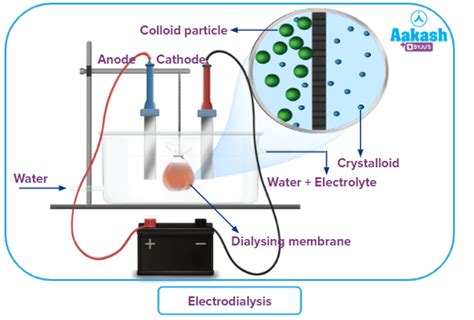
Table of Contents
Dialysis: Separating Solutions from Colloids
Dialysis is a crucial process in various scientific and medical fields, primarily known for its role in treating kidney failure. However, its underlying principle—the separation of dissolved substances (solutes) based on size—extends far beyond this crucial medical application. This article delves into the detailed mechanism of dialysis, specifically focusing on its ability to separate solutions from colloids. We'll explore the underlying principles, the practical applications, and the limitations of this powerful separation technique.
Understanding Solutions and Colloids
Before diving into the mechanics of dialysis, it's vital to understand the key differences between solutions and colloids. Both involve the dispersion of one substance within another, but their characteristics differ significantly:
Solutions:
- Particle size: Solute particles are extremely small, typically less than 1 nanometer (nm) in diameter. They are dissolved at the molecular or ionic level.
- Appearance: Solutions are homogeneous; they appear completely clear and transparent. There's no visible scattering of light.
- Separation: Components of a solution cannot be easily separated by simple physical methods like filtration.
Colloids:
- Particle size: Colloidal particles are much larger than solute particles, ranging from 1 nm to 1000 nm. These particles are dispersed but not dissolved.
- Appearance: Colloids can appear transparent, translucent, or opaque, depending on the size and concentration of the dispersed particles. The Tyndall effect – the scattering of light – is often observed in colloids.
- Separation: Colloidal particles can sometimes be separated using techniques like ultrafiltration or centrifugation, but simple filtration is ineffective.
The Mechanism of Dialysis: Size-Based Separation
Dialysis leverages the difference in particle size between solutions and colloids to achieve separation. It utilizes a semipermeable membrane, a selectively permeable barrier with pores of a specific size. This membrane allows the passage of small solute molecules (from the solution) but restricts the movement of larger colloidal particles.
The process typically involves placing the solution containing both solutes and colloids within a dialysis bag or chamber made from the semipermeable membrane. This bag is then immersed in a large volume of solvent (often distilled water or a specifically formulated dialysis solution), known as the dialysate.
The concentration gradient drives the movement of the smaller solute molecules across the membrane from the solution into the dialysate. This movement continues until equilibrium is reached – where the concentration of the solute is roughly equal on both sides of the membrane. The larger colloidal particles, being unable to pass through the pores of the membrane, remain within the dialysis bag.
Key Factors Affecting Dialysis Efficiency:
Several factors significantly influence the effectiveness of dialysis:
- Membrane pore size: The size of the pores in the semipermeable membrane is critical. Smaller pores enhance the separation of smaller solutes from larger colloids.
- Concentration gradient: A larger concentration difference between the solution and the dialysate accelerates the rate of dialysis.
- Surface area of the membrane: A greater surface area of the membrane allows for more efficient solute exchange.
- Temperature: Higher temperatures generally increase the rate of diffusion, thus speeding up dialysis.
- Stirring: Agitation of the solution and the dialysate helps to minimize concentration polarization near the membrane and enhances the rate of mass transfer.
Applications of Dialysis in Separating Solutions from Colloids
The ability of dialysis to separate solutions from colloids finds widespread applications in diverse fields:
1. Medical Applications: Hemodialysis and Peritoneal Dialysis
The most well-known application is in treating kidney failure. Hemodialysis, a process where a patient's blood is passed through an artificial kidney (dialyzer) containing a semipermeable membrane, removes waste products (solutes) and excess fluid from the blood, while retaining blood cells and proteins (colloids). Peritoneal dialysis utilizes the patient's own peritoneal membrane as the semipermeable barrier. Both techniques rely on the principle of size-based separation to cleanse the blood.
2. Pharmaceutical and Biotechnology Applications
Dialysis plays a crucial role in purifying and concentrating biological molecules like proteins and enzymes. In pharmaceutical manufacturing, it is used to remove impurities, salts, and other small molecules from protein solutions. This purification step is essential to ensure the safety and efficacy of therapeutic proteins.
3. Analytical Chemistry
Dialysis finds application in analytical chemistry to separate and purify samples for various analytical techniques. For example, it can be used to remove interfering substances from a sample before analysis, thereby improving the accuracy and reliability of the results.
4. Food and Beverage Industry
Dialysis can be employed in the food industry to remove unwanted components, such as salts or unwanted sugars, from various food products. This can improve the taste, texture, and shelf life of the products. Moreover, it can be used to concentrate valuable components like proteins or flavors.
5. Environmental Monitoring
Dialysis is used in environmental monitoring to separate and concentrate pollutants or contaminants from water or soil samples. This allows for more sensitive and accurate detection of pollutants and helps in assessing the environmental impact of various factors.
Limitations of Dialysis
Despite its numerous advantages, dialysis has limitations:
- Membrane fouling: The build-up of solutes and other substances on the membrane surface can reduce its permeability and efficiency. Regular cleaning or replacement of the membrane is often necessary.
- Slow process: Dialysis can be a time-consuming process, especially for large volumes or high concentrations of solutes.
- Membrane selectivity: Achieving complete separation can be challenging, especially when the size difference between solutes and colloids is small. Some solute molecules might pass through the membrane even if they are relatively large.
- Cost: The equipment and materials required for dialysis can be expensive, making it less accessible in some settings.
Advanced Dialysis Techniques
Researchers continue to develop and refine dialysis techniques to overcome some of these limitations:
- Ultrafiltration: A modified form of dialysis that applies pressure to accelerate the removal of solutes and water.
- Diafiltration: A process that combines dialysis with continuous addition of fresh dialysate to improve the efficiency of solute removal.
- Electrodialysis: Uses an electric field to enhance the separation of charged molecules.
Conclusion: The Versatility of Dialysis
Dialysis, a simple yet powerful separation technique, plays a crucial role in numerous scientific and medical applications. Its ability to separate solutions from colloids based on size-exclusion is fundamental to its utility. While limitations exist, ongoing research and development continue to improve the efficiency and applications of dialysis, solidifying its position as a vital tool in various fields. From the life-saving treatment of kidney failure to the purification of biological molecules, dialysis remains a cornerstone of modern technology. Understanding its mechanism, applications, and limitations is crucial for anyone involved in fields utilizing this powerful separation technique.
Latest Posts
Latest Posts
-
What Is The Opposite Of Sublimation
Mar 17, 2025
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Dialysis Can Be Used To Separate Solutions From Colloids. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
