Difference Between Ionic And Molecular Compounds Examples
Muz Play
Mar 16, 2025 · 6 min read
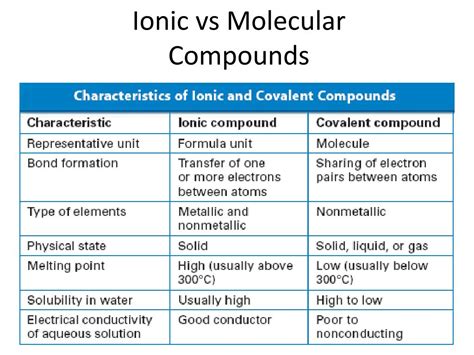
Table of Contents
Delving Deep into the Differences Between Ionic and Molecular Compounds: A Comprehensive Guide
Understanding the fundamental differences between ionic and molecular compounds is crucial for anyone studying chemistry. These differences stem from the nature of the bonds holding the atoms together – ionic bonds versus covalent bonds – leading to vastly different properties. This comprehensive guide will explore these differences in detail, providing numerous examples to solidify your understanding.
What are Ionic Compounds?
Ionic compounds are formed through the electrostatic attraction between oppositely charged ions. These ions are created when atoms transfer electrons – a process known as electron transfer or ionic bonding. Typically, this occurs between a metal and a non-metal. The metal atom loses one or more electrons to become a positively charged ion (cation), while the non-metal atom gains these electrons to become a negatively charged ion (anion). The resulting strong electrostatic force of attraction holds these ions together in a crystal lattice structure.
Key Characteristics of Ionic Compounds:
- High melting and boiling points: The strong electrostatic forces require a significant amount of energy to overcome, resulting in high melting and boiling points.
- Crystalline structure: Ionic compounds form ordered, three-dimensional crystal lattices. This contributes to their solid nature at room temperature.
- Solubility in polar solvents: Many ionic compounds readily dissolve in polar solvents like water because the polar water molecules can interact with the charged ions, effectively separating them.
- Electrical conductivity: Ionic compounds conduct electricity when molten or dissolved in water, as the ions become mobile and can carry an electric current. They do not conduct electricity in their solid state because the ions are fixed in the crystal lattice.
- Hardness and Brittleness: Ionic compounds are generally hard but brittle. Applying pressure can misalign the layers of ions, leading to repulsion and fracturing.
Examples of Ionic Compounds:
Let's illustrate with some common examples:
- Sodium chloride (NaCl): Table salt. Sodium (Na) loses one electron to become Na⁺, and chlorine (Cl) gains one electron to become Cl⁻.
- Magnesium oxide (MgO): Magnesium (Mg) loses two electrons to become Mg²⁺, and oxygen (O) gains two electrons to become O²⁻.
- Potassium iodide (KI): Potassium (K) loses one electron to become K⁺, and iodine (I) gains one electron to become I⁻.
- Calcium carbonate (CaCO₃): Found in limestone and marble. Calcium (Ca) forms Ca²⁺ ions, and carbonate (CO₃)²⁻ is a polyatomic anion.
- Aluminum oxide (Al₂O₃): Aluminum (Al) forms Al³⁺ ions, and oxygen (O) forms O²⁻ ions. This compound is very hard and has a high melting point, making it suitable for applications like abrasives.
What are Molecular Compounds?
Molecular compounds, also known as covalent compounds, are formed by the sharing of electrons between atoms. This sharing creates a covalent bond, where atoms are held together by the mutual attraction of their nuclei to the shared electrons. Molecular compounds are typically formed between non-metal atoms.
Key Characteristics of Molecular Compounds:
- Lower melting and boiling points: The covalent bonds are generally weaker than ionic bonds, resulting in lower melting and boiling points. Many molecular compounds are liquids or gases at room temperature.
- Varied physical states: Molecular compounds can exist as solids, liquids, or gases at room temperature depending on the strength of the intermolecular forces (forces between molecules).
- Solubility varies: Solubility in water and other solvents varies greatly depending on the polarity of the molecule. Polar molecules tend to dissolve in polar solvents, while non-polar molecules dissolve in non-polar solvents.
- Poor electrical conductivity: Molecular compounds generally do not conduct electricity because they do not have freely moving charged particles.
- Lower Hardness: Molecular compounds are generally softer than ionic compounds.
Examples of Molecular Compounds:
Here are some illustrative examples:
- Water (H₂O): Oxygen shares electrons with two hydrogen atoms. The resulting molecule is polar due to the difference in electronegativity between oxygen and hydrogen.
- Carbon dioxide (CO₂): Carbon shares electrons with two oxygen atoms. This molecule is linear and non-polar.
- Methane (CH₄): Carbon shares electrons with four hydrogen atoms. This molecule is tetrahedral and non-polar.
- Ammonia (NH₃): Nitrogen shares electrons with three hydrogen atoms. This molecule is pyramidal and polar.
- Ethanol (C₂H₅OH): A common alcohol. Contains carbon, hydrogen, and oxygen atoms bonded covalently. Polar due to the hydroxyl (-OH) group.
- Glucose (C₆H₁₂O₆): A simple sugar. A large molecule with many covalent bonds. Polar due to multiple hydroxyl groups.
- Benzene (C₆H₆): An aromatic hydrocarbon. Contains a ring of carbon atoms with alternating single and double bonds. Non-polar.
Comparing Ionic and Molecular Compounds: A Table Summary
| Feature | Ionic Compounds | Molecular Compounds |
|---|---|---|
| Bonding | Ionic (electron transfer) | Covalent (electron sharing) |
| Melting Point | High | Low |
| Boiling Point | High | Low |
| Solubility | Usually soluble in polar solvents | Varies depending on polarity |
| Conductivity | Conducts electricity when molten or in solution | Generally does not conduct electricity |
| Hardness | Hard and brittle | Generally softer |
| Structure | Crystalline lattice | Molecular structure (discrete molecules) |
| Formation | Between metal and non-metal | Between non-metals |
Distinguishing Between Ionic and Molecular Compounds: Practical Tips
Identifying whether a compound is ionic or molecular often relies on understanding the elements involved. Metals tend to form cations and participate in ionic bonds, while non-metals tend to share electrons and participate in covalent bonds. However, there are exceptions and nuances. Consider the following:
- Examine the elements: If the compound is composed of a metal and a non-metal, it's highly likely to be ionic. If it's composed entirely of non-metals, it's likely to be molecular.
- Consider electronegativity differences: A significant difference in electronegativity between atoms suggests ionic bonding. A smaller difference indicates covalent bonding. However, very small differences can still lead to polar covalent bonds.
- Observe physical properties: High melting and boiling points, hardness, and solubility in polar solvents suggest ionic character. Low melting and boiling points, and lack of conductivity suggest molecular character.
Beyond the Basics: Intermediate Concepts
The distinction between ionic and molecular compounds isn't always clear-cut. Some compounds exhibit characteristics of both types. Here are some advanced concepts to consider:
- Polar Covalent Bonds: In covalent bonds, if the atoms involved have different electronegativities, the shared electrons will be pulled closer to the more electronegative atom. This creates a polar bond, where one end of the bond is slightly positive and the other is slightly negative. This polarity affects the overall properties of the molecule.
- Polyatomic Ions: These are groups of atoms bonded covalently that carry a net electric charge. They act as single units in ionic compounds. Examples include sulfate (SO₄²⁻), nitrate (NO₃⁻), and ammonium (NH₄⁺).
- Network Covalent Compounds: These are substances where covalent bonds extend throughout a vast network of atoms, creating a giant molecule. Diamond and silicon dioxide (SiO₂) are examples. These compounds tend to have extremely high melting points and hardness.
- Metallic Bonding: This type of bonding is found in metals, where electrons are delocalized and shared among many atoms. This accounts for the high conductivity and malleability of metals.
Conclusion
Understanding the differences between ionic and molecular compounds is essential for comprehending the diverse properties of matter. While the simple metal-nonmetal distinction is a useful starting point, delving deeper into electronegativity differences, polyatomic ions, and various bonding types reveals the nuances and complexities of chemical bonding. By applying the principles outlined in this guide, you can effectively analyze and predict the properties of various compounds and gain a more comprehensive grasp of chemistry.
Latest Posts
Latest Posts
-
What Is The Opposite Of Sublimation
Mar 17, 2025
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Ionic And Molecular Compounds Examples . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
