Different Conformations Of The Same Molecule
Muz Play
Mar 24, 2025 · 6 min read
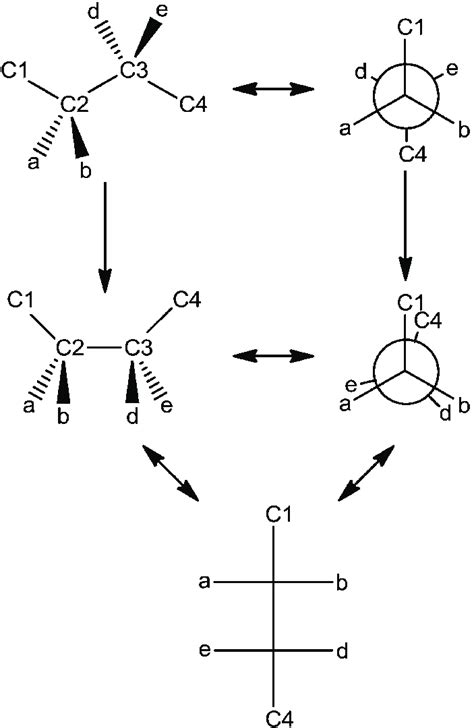
Table of Contents
Different Conformations of the Same Molecule: Exploring Molecular Flexibility
Understanding the diverse ways molecules can twist and turn is fundamental to comprehending their behavior and properties. This article delves into the fascinating world of molecular conformations, exploring the different shapes a single molecule can adopt without breaking or forming any bonds. We'll examine the factors influencing these conformational changes, the methods used to represent them, and the significant impact conformations have across various scientific disciplines.
What are Molecular Conformations?
Molecules are not static entities; they are dynamic structures constantly vibrating and undergoing conformational changes. A conformation refers to the spatial arrangement of atoms in a molecule that can be interconverted by rotation around single bonds. Crucially, these changes don't involve breaking and reforming chemical bonds; instead, they involve adjustments in the angles between atoms. Think of it like twisting a flexible model of a molecule – you're changing its shape, but not breaking any of its parts.
Different conformations of the same molecule are known as conformers or rotamers (specifically for rotamers around single bonds). These conformers can vary significantly in energy, with some being more stable (lower energy) and thus more prevalent than others. The energy differences between conformers arise from factors such as steric hindrance, dipole-dipole interactions, and hydrogen bonding.
Factors Influencing Conformations
Several key factors contribute to the range of conformations a molecule can adopt:
-
Bond Rotation: The most significant factor is the freedom of rotation around single bonds (sigma bonds). Double bonds (pi bonds) exhibit restricted rotation, leading to fewer possible conformations.
-
Steric Hindrance: Bulky substituents attached to a molecule can clash, leading to high-energy, less stable conformations. The molecule will tend to adopt conformations that minimize these steric interactions.
-
Electrostatic Interactions: Interactions between polar groups within a molecule can influence its conformation. For example, dipole-dipole attractions can stabilize certain conformations, while repulsions between similarly charged groups can destabilize others.
-
Hydrogen Bonding: Hydrogen bonding, a strong intermolecular force, can significantly influence molecular conformation, particularly in biological molecules like proteins and nucleic acids.
-
Solvent Effects: The surrounding solvent can also play a role. Polar solvents might stabilize conformations with exposed polar groups, while nonpolar solvents might favor conformations that minimize contact with the solvent.
Representing Molecular Conformations
Several methods are used to visually represent and analyze molecular conformations:
-
Newman Projections: This simple yet powerful technique projects the molecule along a carbon-carbon bond, showing the front carbon as a circle and the back carbon as a point. It clearly illustrates the dihedral angles between substituents.
-
Sawhorse Projections: These show the molecule in a three-dimensional perspective, allowing for a more intuitive visualization of the spatial arrangement of atoms.
-
Fischer Projections: This type of projection simplifies the representation of chiral centers in molecules, particularly useful in carbohydrate chemistry. However, it doesn't always fully capture the three-dimensional nature of conformations.
-
Molecular Models: Physical models, both ball-and-stick and space-filling, provide a tangible representation of molecular structure and facilitate visualization of conformational changes. These are especially helpful for understanding complex molecules and steric effects.
-
Computer-Aided Molecular Modeling: Sophisticated computational software is essential for simulating and analyzing conformational changes in large, complex molecules. This allows researchers to explore the energy landscape of conformations and identify the most stable structures. Techniques like molecular dynamics and Monte Carlo simulations are frequently employed.
Examples of Different Conformations
Let's examine specific examples to illustrate the concept:
1. Butane (C₄H₁₀)
Butane is a simple hydrocarbon with two conformations of particular interest:
-
Anti-conformation: In this conformation, the two methyl groups (CH₃) are positioned 180° apart. This minimizes steric interactions and represents the most stable conformation.
-
Gauche-conformation: Here, the methyl groups are positioned 60° apart. This conformation experiences steric crowding, resulting in higher energy and reduced stability compared to the anti-conformation. Two gauche conformers exist, and they are mirror images of each other, although they are not enantiomers.
2. Cyclohexane (C₆H₁₂)
Cyclohexane, a six-membered ring, demonstrates the importance of conformational flexibility to reduce ring strain. Its most stable conformation is the chair conformation, where all bond angles are close to ideal tetrahedral angles (109.5°). The boat conformation and twist-boat conformation are higher-energy conformations due to steric strain and torsional strain.
3. Proteins
Proteins are biological macromolecules with intricate three-dimensional structures critical for their function. Their conformations are dictated by a complex interplay of various interactions: peptide bonds (amide bonds), hydrogen bonds (particularly alpha-helices and beta-sheets), disulfide bridges, hydrophobic interactions, and van der Waals forces. The precise conformation of a protein is crucial for its activity, and misfolding can lead to diseases like Alzheimer's and Parkinson's.
4. Nucleic Acids
DNA and RNA, the carriers of genetic information, also exhibit conformational flexibility. The double helix structure of DNA is stabilized by hydrogen bonding between complementary base pairs, and its conformation can vary depending on environmental factors like hydration and ionic strength. RNA molecules adopt diverse structures, ranging from simple hairpins to complex three-dimensional folds essential for their function as enzymes and regulatory molecules.
The Significance of Conformational Analysis
Understanding molecular conformations has profound implications across various scientific fields:
-
Drug Design: Conformational analysis is crucial in drug discovery. The active conformation of a drug molecule needs to fit precisely into the target binding site (receptor) to exert its therapeutic effect. Understanding the conformational landscape of potential drug candidates is essential for optimizing their efficacy and reducing side effects.
-
Materials Science: The properties of polymers and other materials are highly dependent on their conformational arrangements. Manipulating the conformations of these materials allows researchers to tailor their properties for specific applications. This includes controlling their strength, elasticity, and optical characteristics.
-
Biochemistry: Conformational changes in biological macromolecules are fundamental to their function. Enzymes, for example, often undergo significant conformational changes upon binding substrates, enabling them to catalyze reactions. Understanding these conformational changes is key to deciphering their mechanisms of action.
-
Spectroscopy: Different conformations often exhibit distinct spectroscopic signatures (e.g., NMR, IR, Raman). By analyzing these spectroscopic data, researchers can obtain detailed information about the conformational distribution and dynamics of molecules.
Conclusion: A Dynamic World of Molecular Shapes
Molecular conformations are a dynamic aspect of chemistry, influencing various properties and behaviors of molecules. Understanding the factors that determine conformations and the techniques employed to analyze them is essential across multiple scientific domains. From the simple rotation around single bonds to the intricate folding of proteins, the ability to predict and manipulate molecular conformations opens up exciting possibilities in fields such as drug discovery, materials science, and biochemistry. The ongoing research in this area continues to reveal the rich complexity and profound implications of molecular flexibility.
Latest Posts
Latest Posts
-
How To Find Domain And Range Algebraically
Mar 27, 2025
-
Differences Between Sheep And Human Brain
Mar 27, 2025
-
How To Calculate Enthalpy Of Combustion
Mar 27, 2025
-
Are All Living Things Composed Of Cells
Mar 27, 2025
-
Solving Systems Of Equations By Substitution Answer Key
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Different Conformations Of The Same Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
