How To Calculate Enthalpy Of Combustion
Muz Play
Mar 27, 2025 · 6 min read
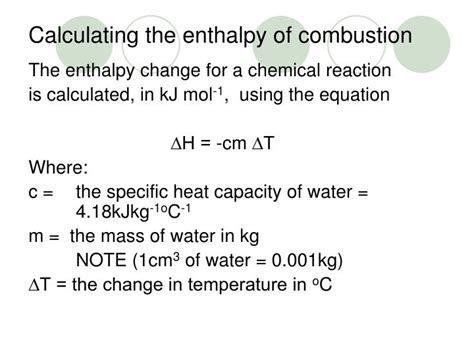
Table of Contents
How to Calculate the Enthalpy of Combustion: A Comprehensive Guide
Enthalpy of combustion, denoted as ΔH<sub>c</sub>, represents the heat released during the complete combustion of one mole of a substance. Understanding how to calculate this crucial thermodynamic property is vital in various fields, from chemistry and chemical engineering to environmental science and energy production. This comprehensive guide will delve into the methods for calculating enthalpy of combustion, exploring both experimental and theoretical approaches.
Understanding Enthalpy of Combustion
Before delving into the calculations, let's solidify our understanding of the concept. Combustion is a rapid exothermic redox reaction between a substance and an oxidant, typically oxygen. The enthalpy change, ΔH<sub>c</sub>, is negative because heat is released to the surroundings. The complete combustion of a hydrocarbon, for instance, produces carbon dioxide and water as the primary products.
The magnitude of ΔH<sub>c</sub> is highly dependent on the chemical structure of the substance. Factors influencing it include:
- Bond energies: Stronger bonds require more energy to break, affecting the overall energy released during combustion.
- Molecular structure: Branched alkanes generally have lower enthalpies of combustion compared to their straight-chain isomers due to differences in stability.
- Resonance stabilization: Compounds with resonance structures (like benzene) often exhibit lower enthalpies of combustion due to increased stability.
Methods for Calculating Enthalpy of Combustion
There are two primary methods for determining the enthalpy of combustion:
- Experimental Determination using Calorimetry: This method involves measuring the heat released during a combustion reaction using a calorimeter. Bomb calorimetry is a common technique used for this purpose.
- Theoretical Calculation using Hess's Law and Standard Enthalpies of Formation: This approach utilizes standard thermodynamic data to calculate the enthalpy of combustion indirectly.
Let's explore each method in detail.
1. Experimental Determination using Calorimetry
Bomb calorimetry is the most accurate experimental method for determining the enthalpy of combustion. This technique involves burning a precisely weighed sample of the substance in a sealed, oxygen-filled bomb immersed in a water bath. The heat released by the combustion reaction increases the temperature of the water bath, and this temperature change is used to calculate the enthalpy of combustion.
Key Steps Involved:
-
Sample Preparation: A precisely weighed sample of the substance is placed in the bomb calorimeter.
-
Oxygen Filling: The bomb is filled with a high-pressure oxygen atmosphere to ensure complete combustion.
-
Ignition: The sample is ignited using an electrical current.
-
Temperature Measurement: The temperature change of the water bath is meticulously monitored using a thermometer or other temperature-sensing device.
-
Calibration: The calorimeter is calibrated using a substance with a known enthalpy of combustion (e.g., benzoic acid).
-
Calculation: The enthalpy of combustion is calculated using the following formula:
ΔH<sub>c</sub> = -q<sub>water</sub> / n
where:
- ΔH<sub>c</sub> is the enthalpy of combustion (kJ/mol)
- q<sub>water</sub> is the heat absorbed by the water bath (kJ) (calculated as: q<sub>water</sub> = m<sub>water</sub> * C<sub>water</sub> * ΔT)
- m<sub>water</sub> = mass of water
- C<sub>water</sub> = specific heat capacity of water (approximately 4.18 J/g°C)
- ΔT = change in temperature of the water bath
- n is the number of moles of the substance combusted.
Important Considerations:
- Heat capacity of the calorimeter: The heat capacity of the calorimeter itself must be considered in the calculations, as it also absorbs some of the heat released during combustion. This is usually included in the calibration step.
- Incomplete combustion: Incomplete combustion can lead to inaccurate results, so careful attention must be paid to ensure complete oxygen supply and efficient mixing.
- Heat loss: Heat loss to the surroundings can affect the accuracy of the measurements. Proper insulation and experimental techniques are crucial to minimize heat loss.
2. Theoretical Calculation using Hess's Law and Standard Enthalpies of Formation
Hess's Law states that the enthalpy change for a reaction is independent of the pathway taken. This means that the enthalpy change for a reaction can be calculated by summing the enthalpy changes for a series of reactions that add up to the overall reaction. This is particularly useful for calculating the enthalpy of combustion using standard enthalpies of formation (ΔH<sub>f</sub>°).
The standard enthalpy of formation is the enthalpy change when one mole of a substance is formed from its constituent elements in their standard states (usually at 25°C and 1 atm). These values are readily available in thermodynamic data tables.
Steps for Calculating ΔH<sub>c</sub> using Hess's Law:
-
Write the balanced combustion reaction: This involves writing a balanced chemical equation for the complete combustion of the substance. For example, the combustion of methane (CH<sub>4</sub>) is:
CH<sub>4</sub>(g) + 2O<sub>2</sub>(g) → CO<sub>2</sub>(g) + 2H<sub>2</sub>O(l)
-
Obtain standard enthalpies of formation: Look up the standard enthalpies of formation (ΔH<sub>f</sub>°) for each substance in the reaction from a reliable thermodynamic data table. Remember that the standard enthalpy of formation for an element in its standard state is zero.
-
Apply Hess's Law: Use the following equation to calculate the enthalpy of combustion:
ΔH<sub>c</sub>° = Σ [ΔH<sub>f</sub>°(products)] - Σ [ΔH<sub>f</sub>°(reactants)]
This means you sum the standard enthalpies of formation of the products and subtract the sum of the standard enthalpies of formation of the reactants.
Example: Calculating the Enthalpy of Combustion of Methane
Let's assume the following standard enthalpies of formation (values may vary slightly depending on the source):
- ΔH<sub>f</sub>°(CH<sub>4</sub>(g)) = -74.8 kJ/mol
- ΔH<sub>f</sub>°(O<sub>2</sub>(g)) = 0 kJ/mol
- ΔH<sub>f</sub>°(CO<sub>2</sub>(g)) = -393.5 kJ/mol
- ΔH<sub>f</sub>°(H<sub>2</sub>O(l)) = -285.8 kJ/mol
Using Hess's Law:
ΔH<sub>c</sub>° = [ΔH<sub>f</sub>°(CO<sub>2</sub>(g)) + 2ΔH<sub>f</sub>°(H<sub>2</sub>O(l))] - [ΔH<sub>f</sub>°(CH<sub>4</sub>(g)) + 2ΔH<sub>f</sub>°(O<sub>2</sub>(g))]
ΔH<sub>c</sub>° = [(-393.5 kJ/mol) + 2(-285.8 kJ/mol)] - [(-74.8 kJ/mol) + 2(0 kJ/mol)]
ΔH<sub>c</sub>° = -890.1 kJ/mol
This calculation provides the standard enthalpy of combustion for methane.
Sources of Error and Uncertainty
Both experimental and theoretical methods are subject to inherent uncertainties. In calorimetry, heat loss, incomplete combustion, and calibration errors are significant sources of uncertainty. In theoretical calculations, the accuracy depends on the reliability of the standard enthalpy of formation data used. Always report uncertainties associated with your calculated enthalpy of combustion values.
Applications of Enthalpy of Combustion
The enthalpy of combustion has numerous applications, including:
- Energy Production: Determining the energy content of fuels.
- Chemical Engineering: Designing and optimizing combustion processes.
- Environmental Science: Assessing the environmental impact of combustion processes (e.g., greenhouse gas emissions).
- Material Science: Characterizing the thermodynamic properties of new materials.
- Food Science: Determining the caloric content of food.
Conclusion
Calculating the enthalpy of combustion is a crucial task with widespread applications. Both experimental and theoretical methods provide valuable insights into the heat released during combustion. While calorimetry offers a direct experimental measurement, Hess's Law provides a powerful indirect calculation method using readily available thermodynamic data. Understanding these methods and their limitations is essential for accurately determining and interpreting enthalpy of combustion values. Remember to always critically assess the potential sources of error and uncertainty associated with your chosen approach.
Latest Posts
Latest Posts
-
Making A Frequency Table In Excel
Mar 30, 2025
-
Gas To Liquid Endothermic Or Exothermic
Mar 30, 2025
-
Which Best Represents The Center Of The Data Set Below
Mar 30, 2025
-
Which Of The Following Are Ionic Compounds
Mar 30, 2025
-
Bacteria That Require Growth Factors And Complex Nutrients Are Termed
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Enthalpy Of Combustion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
