Diffusion Of Water Across A Selectively Permeable Membrane.
Muz Play
Mar 24, 2025 · 6 min read
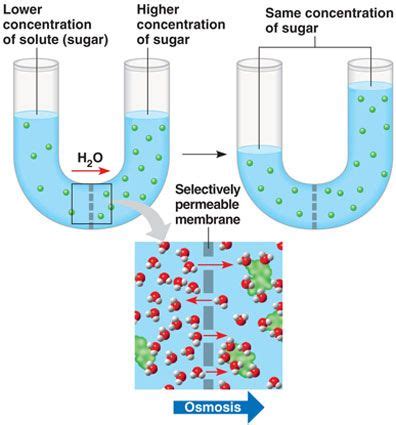
Table of Contents
Diffusion of Water Across a Selectively Permeable Membrane: Osmosis Explained
Understanding how water moves across membranes is fundamental to comprehending numerous biological processes. This intricate movement, driven by the inherent properties of water and the selective nature of cellular membranes, is known as osmosis. This comprehensive article will delve into the intricacies of osmosis, exploring its underlying principles, various applications, and significant implications in biological systems.
What is Osmosis?
Osmosis is the passive transport of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement continues until equilibrium is reached, where the water concentration is equal on both sides of the membrane. The key here is the selectivity of the membrane; it allows water molecules to pass through but restricts the movement of many other molecules and ions. This selective permeability is crucial to maintaining cellular homeostasis and function.
The Role of the Selectively Permeable Membrane
The selectively permeable membrane acts as a gatekeeper, controlling the passage of substances. This membrane is typically composed of a phospholipid bilayer, with embedded proteins that facilitate transport. Water molecules, being small and uncharged, can pass through the lipid bilayer via simple diffusion. However, larger molecules or charged ions require the assistance of protein channels or carriers for transport across the membrane. The precise composition and structure of these membranes vary depending on the specific cell type and its function.
Osmotic Pressure: The Driving Force
The driving force behind osmosis is osmotic pressure. This pressure is the amount of pressure required to prevent the net movement of water across a selectively permeable membrane. The higher the difference in water concentration across the membrane, the greater the osmotic pressure. It's important to remember that osmotic pressure is not a pressure within the solution, but rather the pressure required to counteract the movement of water driven by the concentration gradient.
Understanding Water Potential
Often, the concept of water potential is used to describe the movement of water. Water potential is the tendency of water to move from one area to another. It is influenced by several factors, including solute concentration (more solute means lower water potential) and pressure potential (pressure applied to the solution). Water always moves from an area of higher water potential to an area of lower water potential. Understanding water potential provides a more precise way to quantify and predict water movement in various systems.
Types of Osmotic Solutions
When comparing the concentration of solutes in a solution to the concentration inside a cell, we use terms like isotonic, hypotonic, and hypertonic to describe the solutions.
Isotonic Solution
An isotonic solution has the same solute concentration as the cell's cytoplasm. In this case, there is no net movement of water across the membrane; water moves in and out at equal rates, maintaining the cell's size and shape. This is an ideal environment for many cells.
Hypotonic Solution
A hypotonic solution has a lower solute concentration than the cell's cytoplasm. This means it has a higher water concentration. As a result, water moves into the cell by osmosis. In animal cells, this can lead to lysis (cell bursting) due to excessive water intake. Plant cells, however, have a cell wall that provides structural support, preventing lysis. The cell becomes turgid, or firm, due to the increased internal pressure. This turgor pressure is essential for maintaining plant cell structure and function.
Hypertonic Solution
A hypertonic solution has a higher solute concentration than the cell's cytoplasm, meaning it has a lower water concentration. Water moves out of the cell by osmosis. This causes the cell to crenate or shrivel in animal cells, as the cell loses water and shrinks. In plant cells, the cytoplasm shrinks away from the cell wall, a process called plasmolysis. This can severely impair cell function.
Applications of Osmosis
Osmosis is not merely a theoretical concept; it has numerous practical applications across various fields.
Water Purification
Reverse osmosis is a widely used technique for water purification. This process involves applying pressure to a solution to force water across a semipermeable membrane, leaving behind impurities like salts and other dissolved substances. Reverse osmosis is used in various settings, including homes, industries, and desalination plants to provide clean and safe drinking water.
Food Preservation
Osmosis plays a role in food preservation techniques such as pickling and salting. The high concentration of salt or sugar in the preserving solution draws water out of microorganisms, inhibiting their growth and preventing food spoilage. This reduces the water activity, which is crucial for preventing microbial growth.
Medical Applications
Osmosis is crucial in several medical applications. Intravenous fluids are carefully formulated to be isotonic with blood to prevent damage to red blood cells. Osmosis also influences the absorption of drugs and nutrients in the body. Understanding osmotic pressure is essential in dialysis treatments for patients with kidney failure.
Osmosis in Biological Systems
Osmosis is fundamental to various biological processes in plants and animals.
Water Uptake in Plants
Plants rely heavily on osmosis to absorb water from the soil. Water moves from the soil (high water potential) into the root cells (lower water potential) and then through the xylem vessels to the rest of the plant. This process is critical for plant growth and survival. The turgor pressure created by water uptake in plant cells contributes to the overall structural support of the plant.
Maintaining Cell Volume and Shape
Osmosis is essential in maintaining the appropriate volume and shape of cells. Cells constantly interact with their environment, and the movement of water across their membranes is crucial for maintaining homeostasis. Disruptions in osmotic balance can lead to cell damage or death.
Maintaining Blood Pressure
In animals, osmosis plays a critical role in maintaining blood pressure and electrolyte balance. The kidneys regulate water and solute concentration in the blood through selective reabsorption and excretion. These processes are essential for maintaining blood pressure within a healthy range and preventing dehydration.
Factors Affecting Osmosis
Several factors influence the rate of osmosis.
Temperature
Higher temperatures generally increase the rate of osmosis because water molecules have higher kinetic energy, leading to faster movement across the membrane.
Concentration Gradient
A steeper concentration gradient (larger difference in water concentration across the membrane) results in a faster rate of osmosis.
Surface Area of the Membrane
A larger surface area of the membrane increases the rate of osmosis as more water molecules can pass through simultaneously.
Membrane Permeability
The permeability of the membrane to water also affects the rate of osmosis; a more permeable membrane allows for faster water movement.
Conclusion
Osmosis is a vital process that underpins many biological and industrial applications. Understanding the principles of osmosis, including the role of the selectively permeable membrane, osmotic pressure, water potential, and the effects of isotonic, hypotonic, and hypertonic solutions, is crucial for appreciating the complexities of biological systems and for developing numerous technological applications. From water purification to medical treatments, osmosis continues to be a cornerstone of scientific advancements and technological progress. Further research continues to unravel the intricate details of this fundamental biological process and explore its potential in diverse fields. The ongoing study of osmosis contributes to a deeper understanding of life at the cellular level and allows for improvements in many areas impacting our everyday lives.
Latest Posts
Latest Posts
-
What Is Damping In A Wave
Mar 28, 2025
-
How To Calculate Heat Of Dissolution Without Temperature
Mar 28, 2025
-
Which Part Of The Brain Contains The Cardiac Control Center
Mar 28, 2025
-
The Lewis Diagram Below Represents An Aluminum Ion Value
Mar 28, 2025
-
Conditional Probability And The Multiplication Rule
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Diffusion Of Water Across A Selectively Permeable Membrane. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
