The Lewis Diagram Below Represents An Aluminum Ion. Value
Muz Play
Mar 28, 2025 · 6 min read
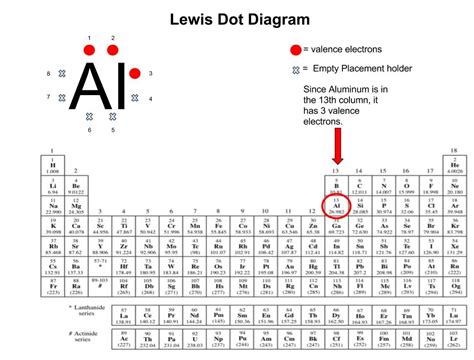
Table of Contents
The Lewis Diagram Below Represents an Aluminum Ion: Value and Significance in Chemistry
The Lewis diagram, a cornerstone of introductory chemistry, provides a simplified yet powerful visualization of an atom's valence electrons and bonding behavior. Understanding Lewis diagrams is crucial for predicting molecular geometry, reactivity, and various chemical properties. This article delves deep into the representation of an aluminum ion using a Lewis diagram, exploring its value, significance, and implications within the broader context of chemical bonding and reactivity.
Understanding Lewis Diagrams: A Foundation for Chemical Visualization
Before focusing on the aluminum ion, let's solidify our understanding of Lewis diagrams themselves. Developed by Gilbert N. Lewis, these diagrams use dots to represent valence electrons – the electrons in the outermost shell of an atom that participate in chemical bonding. The nucleus and inner electrons are not explicitly shown, as their involvement in bonding is minimal.
Key Features of a Lewis Diagram:
- Symbol of the Element: The chemical symbol of the element is placed in the center.
- Valence Electrons: Dots are arranged around the symbol, representing the valence electrons. Typically, dots are placed singly around the symbol until all four sides are occupied, then pairing begins. This reflects Hund's rule of maximum multiplicity.
- Octet Rule (Mostly): Many elements strive to achieve a stable electron configuration with eight valence electrons (an octet), resembling the noble gas configuration. However, exceptions exist, especially with transition metals and elements beyond the second period.
Representing Aluminum (Al) and its Ion (Al³⁺)
Aluminum, with an atomic number of 13, possesses an electron configuration of 1s²2s²2p⁶3s²3p¹. This means it has three valence electrons located in the 3s and 3p orbitals. The Lewis diagram for a neutral aluminum atom would therefore show the aluminum symbol (Al) surrounded by three dots:
.
. Al .
.
However, aluminum is highly reactive and readily loses its three valence electrons to achieve a stable, noble gas configuration resembling neon (1s²2s²2p⁶). This process results in the formation of a triply positively charged aluminum ion, denoted as Al³⁺.
The Lewis diagram for an Al³⁺ ion is simply:
Al
There are no dots because all three valence electrons have been lost. This represents a completely filled electron shell beneath the valence shell, resulting in exceptional stability.
Value and Significance of the Al³⁺ Lewis Diagram
The simplicity of the Al³⁺ Lewis diagram belies its importance in understanding several key aspects of aluminum chemistry:
1. Predicting Reactivity:
The absence of valence electrons in the Al³⁺ Lewis diagram clearly demonstrates its inertness compared to the neutral aluminum atom. The Al³⁺ ion is significantly less reactive because it has achieved a stable electron configuration. It no longer has the capacity to readily form covalent bonds by sharing electrons or ionic bonds by transferring electrons.
2. Understanding Ionic Bonding:
The Al³⁺ Lewis diagram is essential for visualizing ionic bonding. Aluminum's tendency to lose three electrons to form Al³⁺ highlights its role as a cation in ionic compounds. These cations are electrostatically attracted to anions (negatively charged ions) to form stable ionic lattices. For example, in aluminum oxide (Al₂O₃), Al³⁺ ions are surrounded by O²⁻ ions in a strong, crystal lattice structure.
3. Explaining Chemical Properties:
Many chemical properties of aluminum compounds can be attributed to the presence of the Al³⁺ ion. For example, the high melting and boiling points of many aluminum compounds are due to the strong electrostatic attraction between the Al³⁺ cations and their counterions. Furthermore, the behavior of aluminum compounds in aqueous solutions can often be rationalized by considering the hydration of the Al³⁺ ion.
4. Predicting Coordination Chemistry:
The Al³⁺ ion, due to its positive charge and its empty valence orbitals, is a Lewis acid; it readily accepts electron pairs from Lewis bases. This ability is crucial in coordination chemistry where the Al³⁺ ion forms complexes with ligands (molecules or ions that donate electron pairs). The structure and reactivity of these complexes can be understood by considering the Lewis diagram of Al³⁺ and the electron-donating ability of the ligands.
5. Applications in Materials Science:
Aluminum and its compounds find extensive applications in various materials due to the unique characteristics imparted by the Al³⁺ ion. For instance, the strong ionic bonds in aluminum oxide (Al₂O₃), a result of the interaction between Al³⁺ and O²⁻, contribute to its high hardness and refractoriness. Aluminum alloys, often employed in aerospace and automotive industries, owe their strength and lightweight properties to the metallic bonding involving the Al³⁺ ion along with other metallic elements.
Beyond the Basics: Considering Limitations and Extensions
While the Lewis diagram for Al³⁺ is a valuable tool, it has some limitations.
1. Simplicity vs. Reality:
The Lewis diagram simplifies the complex electron distribution in an ion. It doesn't account for electron orbital shapes and energies in detail. It also omits the influence of surrounding ions and molecules in real chemical environments. More sophisticated methods like molecular orbital theory are necessary for a more accurate representation of electron distribution in real molecules and ions.
2. Limitations of the Octet Rule:
Although the Al³⁺ ion satisfies the pseudo-noble gas configuration, this isn't always the case for all metal ions. Many transition metals form ions with incomplete octets. The Lewis diagram alone is inadequate to predict the behavior of these more complex ions.
3. Coordination Complexes:
While the Al³⁺ Lewis diagram helps illustrate the acceptance of electron pairs in coordination complexes, it doesn't predict the precise geometry of these complexes. Detailed information about ligand field theory and crystal field theory is needed for a better understanding of coordination geometry and stability.
4. Beyond Simple Ions:
The Lewis diagram is most effective for simple ions like Al³⁺. For larger, more complex molecules and ions, the Lewis diagrams can become cumbersome and may not accurately reflect the true bonding and electronic structure.
Conclusion: The Enduring Value of Simplicity
The Lewis diagram for the aluminum ion (Al³⁺), despite its simplicity, remains a valuable and indispensable tool in chemistry. Its ability to effectively illustrate the valence electron configuration and the formation of ionic bonds significantly contributes to understanding aluminum's reactivity, chemical properties, and its role in various applications. Although more sophisticated methods provide a deeper understanding of the complexities of chemical bonding, the Lewis diagram provides a fundamental framework for visualizing and interpreting the behavior of simple ions, making it an essential part of any chemist's toolkit. Its enduring value lies in its ability to serve as a springboard to more advanced concepts in chemistry, providing a solid foundation for tackling more intricate chemical problems. The simplicity and clarity of the Al³⁺ Lewis diagram make it a powerful tool for visualizing fundamental chemical principles, highlighting the importance of simple models in understanding complex systems.
Latest Posts
Latest Posts
-
How Do You Determine Total Magnification Of A Microscope
Mar 31, 2025
-
Label The Formed Elements Of The Blood
Mar 31, 2025
-
What Is The Smallest Component That Supports Life
Mar 31, 2025
-
Write The Iupac Names Of The Given Carboxylic Acids
Mar 31, 2025
-
Positive Ions Differ From Neutral Atoms In That
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Lewis Diagram Below Represents An Aluminum Ion. Value . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
