Positive Ions Differ From Neutral Atoms In That
Muz Play
Mar 31, 2025 · 6 min read
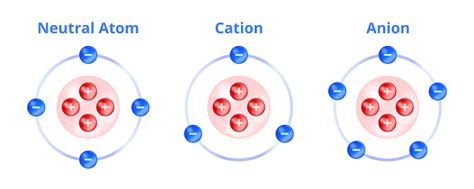
Table of Contents
Positive Ions Differ from Neutral Atoms in That... They've Lost an Electron! A Deep Dive into Ionization
Positive ions, also known as cations, are fundamentally different from neutral atoms. This difference boils down to a single, crucial aspect: the number of electrons. While neutral atoms have an equal number of protons (positive charge) and electrons (negative charge), positive ions possess fewer electrons than protons. This imbalance creates a net positive charge, defining their ionic nature. Understanding this core difference unlocks a world of chemical and physical phenomena. This article will explore this difference in detail, examining the processes leading to ion formation, their properties, and their significance in various fields.
The Fundamental Difference: Electron Count
The most significant distinction between a positive ion and a neutral atom lies in their electron configuration. A neutral atom maintains a balanced electrical charge because the positive charge of its protons in the nucleus is perfectly offset by the negative charge of its orbiting electrons. The number of protons defines the element; for example, a hydrogen atom always has one proton. A neutral hydrogen atom also has one electron.
A positive ion, however, has lost one or more electrons. This loss disrupts the electrical balance, resulting in a net positive charge. For instance, a singly ionized hydrogen ion (H⁺) has lost its single electron, leaving only a proton. Similarly, a sodium ion (Na⁺) has lost one electron from its neutral state, resulting in a net positive charge. The magnitude of the positive charge depends on the number of electrons lost; a doubly ionized ion (e.g., Ca²⁺) has lost two electrons.
The Process of Ionization: How Positive Ions are Formed
The transformation of a neutral atom into a positive ion is a process called ionization. Several mechanisms can induce ionization, including:
1. Electron Removal through Energy Transfer:
This is the most common method. When an atom absorbs sufficient energy—often in the form of heat, light (photons), or collision with another particle—it can overcome the electrostatic attraction holding its electrons. This energy overcomes the ionization energy, the minimum energy needed to remove an electron. The atom then ejects an electron, leaving behind a positively charged ion. This is frequently observed in:
- Gas Discharge Tubes: Applying a high voltage across a gas causes ionization, generating a plasma that emits light. Neon signs are a classic example.
- Flame Tests: Heating metal salts in a flame provides enough energy to ionize the metal atoms, resulting in characteristic colored light emissions.
- Photoionization: High-energy photons (like ultraviolet or X-rays) can directly strip electrons from atoms. This is crucial in processes like the formation of the ionosphere.
2. Chemical Reactions:
During chemical reactions, atoms can transfer electrons to other atoms with higher electron affinity (a measure of an atom's ability to attract electrons). Atoms that readily lose electrons (like alkali metals) often form positive ions in chemical reactions. For example, the reaction between sodium (Na) and chlorine (Cl) to form sodium chloride (NaCl) involves sodium losing an electron to become Na⁺ and chlorine gaining an electron to become Cl⁻.
3. Nuclear Decay:
Certain radioactive decay processes can also lead to ionization. For example, alpha decay involves the emission of an alpha particle (a helium nucleus), leaving behind a positively charged ion from the parent nucleus. This process significantly alters the atomic number and mass number of the atom.
Properties of Positive Ions: How They Differ in Behavior
The loss of electrons dramatically alters the properties of an atom, transforming it into a distinctly different entity:
1. Electrical Charge:
The most fundamental difference is the positive electrical charge. This charge dictates how positive ions interact with electric and magnetic fields, influencing their behavior in various applications such as mass spectrometry and ion chromatography.
2. Size:
Positive ions are generally smaller than their corresponding neutral atoms. This is because the loss of electrons reduces electron-electron repulsion, allowing the remaining electrons to be drawn closer to the nucleus. This size reduction affects their reactivity and ability to form chemical bonds.
3. Chemical Reactivity:
Positive ions are highly reactive due to their incomplete electron shells. They readily participate in chemical reactions to achieve stability, often by forming ionic bonds with negatively charged ions (anions). This drive for stability dictates their chemical behavior and role in many chemical processes.
4. Physical State:
The physical state of a substance can be significantly altered by ionization. For example, neutral sodium is a soft, silvery metal, while sodium ions (Na⁺) exist as part of ionic compounds like sodium chloride (table salt), which is a crystalline solid.
The Significance of Positive Ions: Applications and Importance
Positive ions play vital roles in numerous areas of science and technology:
1. Chemistry:
Understanding the formation and behavior of positive ions is fundamental to chemistry. It is essential for comprehending chemical bonding, reactivity, and the properties of ionic compounds. The periodic table's organization reflects the tendency of elements to form positive ions based on their electron configurations.
2. Biology:
Positive ions, particularly metal ions like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺), are crucial for numerous biological processes. They are involved in nerve impulse transmission, muscle contraction, enzyme activity, and maintaining osmotic balance within cells. Imbalances in ion concentrations can lead to severe physiological problems.
3. Medicine:
Ionizing radiation, though harmful in excessive doses, finds applications in medical imaging (X-rays, CT scans) and cancer therapy (radiation therapy). Furthermore, specific ions are used in medical treatments and diagnostics.
4. Materials Science:
The properties of many materials are directly influenced by the presence of positive ions. For example, the conductivity of metals is related to the mobility of electrons and positive ions within their crystal lattice. The creation of new materials often involves controlling the types and concentrations of positive ions present.
5. Environmental Science:
Positive ions play a crucial role in atmospheric chemistry, influencing air quality and the formation of acid rain. The presence and distribution of ions in water bodies also impact aquatic ecosystems.
6. Analytical Chemistry:
Techniques like mass spectrometry and atomic absorption spectroscopy rely on the detection and analysis of positive ions to identify and quantify elements in various samples.
Conclusion: A World Shaped by Charged Particles
The difference between a positive ion and a neutral atom is a seemingly small one—the loss of one or more electrons. However, this seemingly minor change has profound implications. The resulting positive charge drastically alters the atom's properties, influencing its size, reactivity, and behavior in electric and magnetic fields. This difference underpins a vast array of natural phenomena and technological applications, highlighting the significant role positive ions play in shaping our world, from the biological processes within our bodies to the technologies driving modern advancements. Continued research into the behavior and applications of positive ions will undoubtedly uncover further insights and lead to new innovations across various scientific and technological fields.
Latest Posts
Latest Posts
-
The Most Reactive Group In The Periodic Table
Apr 02, 2025
-
How To Write Quadratic Equation From Graph
Apr 02, 2025
-
How To Place A Condom Catheter
Apr 02, 2025
-
How Many Elements Are Gases At Room Temperature
Apr 02, 2025
-
3 Main Ideas Of Cell Theory
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Positive Ions Differ From Neutral Atoms In That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
