Do Diastereomers Have The Same Physical Properties
Muz Play
Mar 24, 2025 · 5 min read
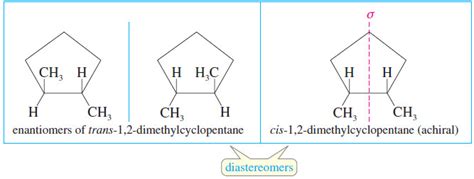
Table of Contents
Do Diastereomers Have the Same Physical Properties? A Deep Dive into Stereochemistry
Diastereomers are a fascinating aspect of organic chemistry, often causing confusion among students and professionals alike. Understanding their properties, particularly whether they share the same physical properties, is crucial for various applications, from drug development to materials science. The short answer is no, diastereomers generally do not have the same physical properties. This article will delve deep into the reasons why, exploring the nuances of stereochemistry and illustrating the differences with clear examples.
Understanding Diastereomers: Beyond Enantiomers
Before we dive into the differences in physical properties, let's establish a firm understanding of what diastereomers are. Isomers, in general, are molecules with the same molecular formula but different structural arrangements. Within isomers, we have several subcategories, including constitutional isomers (different connectivity) and stereoisomers (same connectivity, different spatial arrangement).
Stereoisomers are further divided into two main types:
-
Enantiomers: These are non-superimposable mirror images of each other. Think of your left and right hands – they're mirror images, but you can't perfectly overlay one onto the other. Enantiomers have identical physical properties (except for their interaction with plane-polarized light).
-
Diastereomers: These are stereoisomers that are not mirror images of each other. This is the key distinction. They have different spatial arrangements but are not enantiomers. A molecule with multiple chiral centers will typically have several diastereomers.
Why Diastereomers Exhibit Different Physical Properties
The fundamental reason diastereomers have different physical properties boils down to their unique three-dimensional structures. These structural differences lead to variations in several key characteristics:
-
Melting Point: Diastereomers have different melting points. The arrangement of atoms in space directly influences the intermolecular forces (like van der Waals forces, dipole-dipole interactions, and hydrogen bonding) between molecules. Different intermolecular forces result in different melting points. A tightly packed structure with stronger interactions will generally have a higher melting point than a loosely packed structure with weaker interactions.
-
Boiling Point: Similar to melting points, boiling points also vary among diastereomers. The strength of intermolecular forces plays a critical role here as well. Molecules with stronger interactions require more energy to overcome these forces and transition to the gaseous phase, resulting in higher boiling points.
-
Solubility: Diastereomers often exhibit different solubilities in various solvents. This is because their distinct shapes and polar distributions impact their ability to interact with solvent molecules. A diastereomer with a more polar structure might be more soluble in polar solvents like water, while a less polar diastereomer might prefer nonpolar solvents like hexane.
-
Density: Density, the mass per unit volume, is also affected by the three-dimensional structure. Different packing arrangements of atoms will lead to varying densities. A more compact structure will typically have a higher density than a less compact one.
-
Optical Rotation: While enantiomers rotate plane-polarized light in equal but opposite directions, diastereomers can exhibit different optical rotations, both in magnitude and direction. This is because the asymmetric centers contribute differently to the overall rotation of plane-polarized light.
-
Chromatographic Behavior: Diastereomers can be separated using various chromatographic techniques, such as high-performance liquid chromatography (HPLC) and gas chromatography (GC). This separation is possible due to their differing interactions with the stationary phase in the column. Their distinct polarities and molecular shapes cause them to elute at different times.
-
Spectroscopic Properties: While often subtle, differences can be observed in the NMR (Nuclear Magnetic Resonance) and IR (Infrared) spectra of diastereomers. The chemical shifts in NMR and the vibrational frequencies in IR are sensitive to the local electronic environment and the spatial arrangement of atoms.
Examples Illustrating the Differences
Let's examine a few specific examples to illustrate these differences more concretely:
1. Tartaric Acid: Tartaric acid, a compound found in grapes, has two chiral centers. This results in three stereoisomers: one meso compound (achiral despite having chiral centers) and two enantiomers (D and L tartaric acid). The two enantiomers are mirror images and have identical physical properties (except optical rotation), while the meso-tartaric acid is a diastereomer of both enantiomers and has distinct physical properties from them, notably a different melting point.
2. 2,3-Dibromobutane: This molecule also features two chiral centers and therefore has several stereoisomers including a pair of enantiomers and two diastereomers. These diastereomers will have different melting points, boiling points, solubilities, and other physical properties.
3. 1,2-Dibromocyclohexane: The cis and trans isomers of 1,2-dibromocyclohexane are diastereomers. The cis isomer has both bromine atoms on the same side of the ring, while the trans isomer has them on opposite sides. These isomers differ significantly in their dipole moments, leading to differences in their boiling points and solubilities.
Practical Implications of Diastereomeric Differences
The distinct physical properties of diastereomers have significant implications across various scientific fields:
-
Drug Development: Many drugs are chiral molecules. The different diastereomers of a drug molecule can have drastically different pharmacological activities, potencies, and toxicities. Therefore, it's crucial to synthesize and purify the desired diastereomer for effective and safe drug administration.
-
Material Science: Diastereomers can exhibit different material properties, such as crystallinity, mechanical strength, and optical activity. This makes them valuable in the design and synthesis of novel materials with specific properties.
-
Food Science: Certain food components exist as diastereomers, and their different properties can affect taste, texture, and nutritional value.
-
Analytical Chemistry: The differences in physical properties of diastereomers are exploited in various analytical separation techniques, facilitating the identification and quantification of specific isomers.
Separating Diastereomers: A Challenge and an Opportunity
Unlike enantiomers, which are often difficult to separate, diastereomers can be separated using conventional methods because of their differing physical properties. Techniques such as fractional crystallization, distillation, and chromatography are frequently employed to isolate individual diastereomers. This separation is crucial for applications where the unique properties of specific diastereomers are exploited.
Conclusion: A Deeper Understanding of Molecular Structure and Function
In conclusion, diastereomers, unlike enantiomers, generally do not possess identical physical properties. Their distinct three-dimensional structures lead to differences in melting point, boiling point, solubility, density, optical rotation, chromatographic behavior, and even spectroscopic properties. Understanding these differences is crucial in various fields, from drug development and material science to food science and analytical chemistry. The ability to separate and characterize individual diastereomers opens avenues for developing new drugs, materials, and technologies with tailored properties. The study of diastereomers provides a deeper understanding of the relationship between molecular structure and function, highlighting the importance of stereochemistry in the wider scientific landscape.
Latest Posts
Latest Posts
-
Tipos De Triangulos Segun Sus Angulos
Mar 25, 2025
-
Did The Swahili Coast Require Monsoons To Access
Mar 25, 2025
-
An Irreversible Inhibitor Is One That
Mar 25, 2025
-
When Elements Combine To Form Compounds
Mar 25, 2025
-
Describe The Sampling Distribution Of P Hat
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Do Diastereomers Have The Same Physical Properties . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
