Do Electron Withdrawing Groups Increase Acidity
Muz Play
Mar 31, 2025 · 5 min read
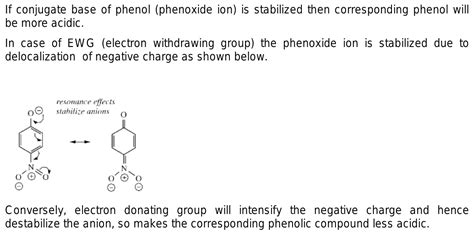
Table of Contents
Do Electron Withdrawing Groups Increase Acidity? A Deep Dive into Organic Chemistry
The question of whether electron withdrawing groups (EWGs) increase acidity is a fundamental concept in organic chemistry. The short answer is a resounding yes, but understanding why requires a deeper exploration of electronic effects, molecular stability, and the very definition of acidity. This article will delve into the intricacies of this relationship, providing a comprehensive understanding supported by examples and explanations.
Understanding Acidity and the Role of Conjugate Bases
Acidity, at its core, is the ability of a molecule to donate a proton (H⁺). The strength of an acid is determined by its willingness to release this proton. This willingness is directly related to the stability of the resulting conjugate base. The more stable the conjugate base, the stronger the acid. This is where electron withdrawing groups play a crucial role.
What are Electron Withdrawing Groups (EWGs)?
EWGs are atoms or groups of atoms that are more electronegative than carbon. They possess a tendency to attract electrons towards themselves, creating a partial positive charge (δ⁺) on the carbon atom they are attached to and a partial negative charge (δ⁻) on themselves. Common examples include:
- Halogens (F, Cl, Br, I): Fluorine is the strongest EWG among the halogens due to its high electronegativity.
- Nitro group (-NO₂): The nitro group is a very strong EWG due to resonance effects.
- Cyano group (-CN): Similar to the nitro group, the cyano group effectively withdraws electron density through resonance.
- Carbonyl group (-C=O): The carbonyl group, found in aldehydes, ketones, carboxylic acids, and esters, is a strong EWG.
- Sulfone group (-SO₂R): The sulfone group is a powerful EWG due to the presence of highly electronegative oxygen atoms and sulfur's ability to participate in resonance.
How EWGs Stabilize Conjugate Bases
When an acid donates a proton, it leaves behind a negatively charged conjugate base. EWGs stabilize this negative charge by:
-
Inductive Effect: EWGs pull electron density away from the negatively charged atom through the sigma bonds, reducing the overall negative charge density and making the conjugate base more stable. This effect diminishes with distance from the EWG.
-
Resonance Effect: Some EWGs, like the nitro and cyano groups, can participate in resonance. This delocalizes the negative charge over multiple atoms, further stabilizing the conjugate base. The more resonance structures that can be drawn, the greater the stabilization.
Let's illustrate this with an example: consider the acidity of acetic acid (CH₃COOH) compared to trifluoroacetic acid (CF₃COOH).
Acetic Acid (CH₃COOH): The conjugate base, acetate ion (CH₃COO⁻), carries a negative charge localized primarily on the carboxylate oxygen.
Trifluoroacetic Acid (CF₃COOH): The conjugate base, trifluoroacetate ion (CF₃COO⁻), has the negative charge dispersed through the inductive effect of the three highly electronegative fluorine atoms. The fluorine atoms pull electron density away from the carboxylate group, stabilizing the negative charge and making trifluoroacetic acid a significantly stronger acid than acetic acid.
Illustrative Examples: Comparing Acid Strengths
Let's examine several examples to solidify the understanding of how EWGs affect acidity.
Carboxylic Acids:
Consider the following series of carboxylic acids:
- Acetic acid (CH₃COOH): No EWGs.
- Chloroacetic acid (ClCH₂COOH): One chlorine atom (EWG).
- Dichloroacetic acid (Cl₂CHCOOH): Two chlorine atoms (EWGs).
- Trichloroacetic acid (Cl₃CCOOH): Three chlorine atoms (EWGs).
The acidity increases progressively as the number of chlorine atoms (strong EWGs) increases. The chlorine atoms inductively withdraw electron density, stabilizing the conjugate base and increasing the acid's strength.
Phenols:
Phenols (ArOH) are weaker acids than carboxylic acids. However, the effect of EWGs on their acidity is still significant. Compare phenol (C₆H₅OH) with para-nitrophenol (p-NO₂C₆H₄OH). The nitro group, a strong EWG, significantly increases the acidity of para-nitrophenol due to its resonance effect. The negative charge on the phenoxide ion is delocalized into the nitro group, stabilizing the conjugate base and increasing acidity.
Alcohols:
Alcohols (ROH) are generally weak acids. Introducing EWGs near the hydroxyl group can enhance their acidity. For example, 2,2,2-trifluoroethanol (CF₃CH₂OH) is a considerably stronger acid than ethanol (CH₃CH₂OH) due to the strong inductive effect of the trifluoromethyl group.
Factors Influencing the Magnitude of the Effect
While EWGs generally increase acidity, the magnitude of the effect depends on several factors:
- Strength of the EWG: Stronger EWGs, like nitro and cyano groups, have a greater impact on acidity than weaker ones, like chlorine.
- Distance from the acidic proton: The inductive effect weakens with distance. EWGs closer to the acidic proton have a more pronounced effect.
- Resonance effects: The ability of an EWG to participate in resonance significantly enhances its effect on acidity.
- Steric effects: In some cases, steric hindrance can influence the ability of an EWG to effectively withdraw electrons, thus affecting the acidity.
Exceptions and Nuances
While the general rule holds true, there are exceptions and nuances to consider. Some EWGs might exert a steric effect that counteracts their electronic effect, reducing their impact on acidity. Additionally, the interplay of inductive and resonance effects can lead to complex outcomes.
Conclusion: A Powerful Relationship
The relationship between electron-withdrawing groups and acidity is a cornerstone of organic chemistry. EWGs enhance acidity by stabilizing the conjugate base through inductive and resonance effects. Understanding this relationship is crucial for predicting and manipulating the reactivity of organic molecules in various chemical processes, synthesis, and analytical techniques. The strength of the effect is influenced by the strength of the EWG, its distance from the acidic proton, and the interplay of inductive and resonance effects, highlighting the complexity and fascinating nature of organic chemistry. The examples provided throughout this article illustrate this fundamental principle, making it a crucial concept for any serious student or practitioner of the field.
Latest Posts
Latest Posts
-
The Most Reactive Group In The Periodic Table
Apr 02, 2025
-
How To Write Quadratic Equation From Graph
Apr 02, 2025
-
How To Place A Condom Catheter
Apr 02, 2025
-
How Many Elements Are Gases At Room Temperature
Apr 02, 2025
-
3 Main Ideas Of Cell Theory
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Do Electron Withdrawing Groups Increase Acidity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
