Do K And Sr Have Similar Properties
Muz Play
Mar 29, 2025 · 6 min read
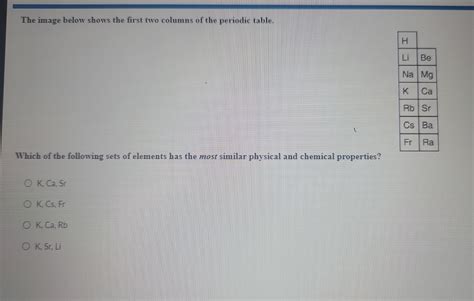
Table of Contents
Do K and Sr Have Similar Properties? A Deep Dive into Alkali and Alkaline Earth Metals
Potassium (K) and Strontium (Sr) are both metallic elements residing relatively close to each other on the periodic table. While their positions hint at shared characteristics, a deeper investigation reveals both striking similarities and crucial differences in their physical and chemical properties. This article will explore these similarities and differences, examining their atomic structure, reactivity, applications, and biological roles. Understanding these nuances is crucial in various fields, including chemistry, materials science, and biology.
Atomic Structure and Electronic Configuration: The Foundation of Similarity
Both potassium and strontium are metals, meaning they readily lose electrons to form positive ions. This fundamental characteristic drives many of their similar properties. Let's examine their atomic structure in detail:
Potassium (K): The Alkali Metal
Potassium, an alkali metal (Group 1), possesses an atomic number of 19. Its electronic configuration is [Ar] 4s¹. This single valence electron in the 4s orbital is easily lost, resulting in a +1 oxidation state. This lone electron contributes significantly to potassium's high reactivity and its tendency to form ionic compounds.
Strontium (Sr): The Alkaline Earth Metal
Strontium, an alkaline earth metal (Group 2), has an atomic number of 38. Its electronic configuration is [Kr] 5s². The two valence electrons in the 5s orbital are also readily lost, forming a +2 oxidation state. While it loses twice as many electrons as potassium, this electron loss still dictates its metallic nature and chemical reactivity.
Similarities in Atomic Structure: The Key to Shared Traits
Both K and Sr have relatively low ionization energies. This means that removing an electron from their outermost shell requires relatively little energy. This low ionization energy is a key factor contributing to their metallic character and reactivity. Both also exhibit metallic bonding, where valence electrons are delocalized, creating a "sea" of electrons that bind the positively charged metal ions together. This explains their characteristic metallic properties like conductivity and malleability.
Chemical Reactivity: A Tale of Two Metals
While both potassium and strontium are reactive metals, their reactivity differs significantly due to their varying number of valence electrons and their atomic radii.
Potassium's Vigorous Reactivity: The Lone Electron's Power
Potassium is exceptionally reactive, readily reacting with water, oxygen, and halogens. Its single valence electron is easily lost, making it a powerful reducing agent. The reaction of potassium with water is highly exothermic, producing hydrogen gas and a significant amount of heat. Exposure to air leads to rapid oxidation, forming potassium oxide (K₂O).
Strontium's Reactivity: A Balanced Act
Strontium is also a reactive metal but less so than potassium. It reacts with water more slowly than potassium, producing strontium hydroxide (Sr(OH)₂) and hydrogen gas. Similarly, its reaction with oxygen is less vigorous, forming strontium oxide (SrO). This lower reactivity is partially attributed to its larger atomic radius and the higher ionization energy required to remove the second valence electron compared to potassium's single electron.
Similarities in Reactivity: The Shared Metallic Nature
Despite differences in intensity, both metals exhibit similar reactivity patterns. They both readily react with non-metals to form ionic compounds, showcasing their tendency to lose electrons to achieve a stable electron configuration. This is a fundamental similarity stemming from their metallic nature and the presence of valence electrons readily available for bonding.
Physical Properties: Density, Melting Point, and More
Potassium and strontium show some similarities and pronounced differences in their physical properties:
Density: A Matter of Atomic Mass and Structure
Strontium is significantly denser than potassium. This difference stems from the higher atomic mass of strontium and a slightly more compact arrangement of atoms in its metallic structure. Density plays a crucial role in their industrial applications and handling.
Melting and Boiling Points: Influence of Metallic Bonding
Both elements have relatively low melting and boiling points compared to many other metals. This is attributed to their metallic bonding, where the relatively weak electrostatic forces between the metal ions and the delocalized electrons require less energy to overcome, leading to lower melting and boiling points. However, strontium has higher melting and boiling points than potassium due to stronger metallic bonds resulting from the higher charge of its ions.
Other Physical Properties: Conductivity and Malleability
Both potassium and strontium are excellent conductors of heat and electricity, typical characteristics of metals. They are also relatively soft and malleable, meaning they can be easily shaped. These properties contribute to their use in various applications, although the degree of malleability differs.
Applications: Exploiting Unique Properties
The distinct properties of potassium and strontium lead to their applications in diverse fields:
Potassium: Essential in Biology and Industry
Potassium plays a vital role in biological systems, being an essential electrolyte for nerve impulse transmission and muscle contraction. Industrially, it's used in fertilizers due to its importance for plant growth. Its reactivity also finds niche applications in certain chemical reactions.
Strontium: From Fireworks to Medical Applications
Strontium is famously used in fireworks to produce brilliant red colors. Its compounds have found applications in refining sugar and in the production of certain alloys. Interestingly, some strontium isotopes are used in medical imaging and radiation therapy.
Similarities in Application Areas: Leveraging Metallic Nature
Although specific applications differ greatly, the underlying metallic nature of both elements facilitates their use in various applications. Their conductivity, for instance, is exploited in different contexts – although rarely directly for both elements in the same applications.
Biological Roles: A Comparison
The biological roles of potassium and strontium differ considerably:
Potassium: An Essential Electrolyte
Potassium is an essential element for all living organisms. It is crucial for maintaining proper fluid balance, nerve impulse transmission, and muscle contraction. Deficiencies can lead to various health problems.
Strontium: A Trace Element with Limited Biological Significance
Strontium's biological role is far less significant than potassium's. It is considered a trace element, and its presence in biological systems is generally much lower. While some studies suggest potential roles in bone metabolism, its biological significance is far less understood and less critical than that of potassium.
Contrasting Biological Roles: A Key Distinction
The contrasting biological roles highlight a fundamental difference between these two elements. Potassium is an essential macronutrient vital for life, whereas strontium plays a much more limited and less crucial role.
Conclusion: Similarities, Differences, and Future Research
Potassium and strontium, while sharing some fundamental similarities as metals, exhibit significant differences in their reactivity, physical properties, applications, and biological roles. Their shared metallic nature underpins their conductivity and malleability. However, the number of valence electrons significantly impacts their reactivity and consequent applications. Further research on strontium's potential applications in biomedicine and materials science could reveal even more of its fascinating properties and potential uses. Understanding these similarities and differences is essential for advancements in various scientific and technological fields. The distinct characteristics of each element make them valuable in unique and diverse ways.
Latest Posts
Latest Posts
-
Lab Skills Using A Graduated Cylinder
Apr 01, 2025
-
Which Molecules In Eukaryotic Cells Regulate Gene Expression
Apr 01, 2025
-
How Do Natural Killer Cells Destroy Invading Pathogens
Apr 01, 2025
-
What Stimulates The Pollen Tube To Grow
Apr 01, 2025
-
How To Find C In Sinusoidal Function
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Do K And Sr Have Similar Properties . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
