Do Liquids Have A Definite Shape
Muz Play
Mar 16, 2025 · 6 min read
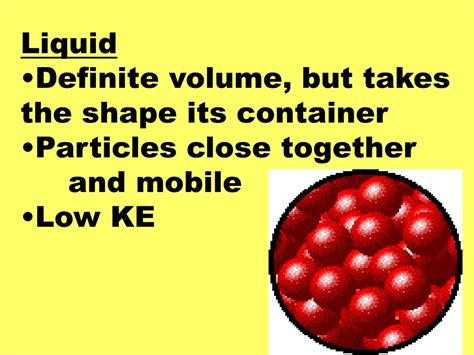
Table of Contents
Do Liquids Have a Definite Shape? Exploring the Properties of Matter
The question of whether liquids have a definite shape is a fundamental concept in understanding the properties of matter. Unlike solids, which possess both a definite shape and volume, liquids exhibit a fascinating interplay between these characteristics. The answer, in short, is no, liquids do not have a definite shape. But understanding why requires delving into the microscopic world of atoms and molecules and exploring the forces that govern their behavior.
Understanding the States of Matter
Before we dive into the specifics of liquids, let's briefly review the three common states of matter: solid, liquid, and gas. Each state is defined by how its constituent particles – atoms and molecules – are arranged and interact.
Solids: Order and Structure
Solids possess a definite shape and volume. Their atoms or molecules are tightly packed in a regular, ordered arrangement, held together by strong intermolecular forces. This rigid structure resists changes in shape and volume, explaining their solidity. Think of a crystal of salt or a block of ice – they maintain their shape unless acted upon by a significant external force.
Liquids: Flow and Adaptability
Liquids, on the other hand, have a definite volume but no definite shape. Their particles are still relatively close together, but they are not fixed in a rigid structure. The intermolecular forces are weaker than in solids, allowing particles to move and slide past each other. This ability to flow is the defining characteristic of liquids. Pour water into a glass, and it takes the shape of the glass. Pour it into a bottle, and it takes the shape of the bottle. The volume remains constant, but the shape conforms to its container.
Gases: Freedom and Expansion
Gases have neither a definite shape nor a definite volume. Their particles are widely dispersed and move freely, with weak intermolecular forces. Gases expand to fill any container they occupy, readily changing both shape and volume in response to their surroundings. Think of air filling a balloon or the perfume spreading across a room.
The Microscopic View: Intermolecular Forces and Particle Movement
The different behaviors of solids, liquids, and gases are fundamentally dictated by the strength of the intermolecular forces between their constituent particles and the amount of kinetic energy these particles possess.
Intermolecular Forces: The Glue that Holds it Together (or Not)
Intermolecular forces are the attractive forces between molecules. These forces are weaker than the intramolecular forces (bonds within a molecule) but still play a crucial role in determining the state of matter. Several types of intermolecular forces exist, including:
- London Dispersion Forces: These are the weakest intermolecular forces and are present in all molecules. They arise from temporary fluctuations in electron distribution, creating temporary dipoles that induce dipoles in neighboring molecules.
- Dipole-Dipole Forces: These forces occur between polar molecules (molecules with a permanent dipole moment). The positive end of one molecule attracts the negative end of another.
- Hydrogen Bonding: This is a special type of dipole-dipole force that occurs when hydrogen is bonded to a highly electronegative atom such as oxygen, nitrogen, or fluorine. Hydrogen bonds are stronger than other dipole-dipole forces.
The strength of these intermolecular forces directly impacts the ability of molecules to move freely. Stronger forces lead to a more rigid structure (solid), while weaker forces allow for greater mobility (liquid and gas).
Kinetic Energy: The Energy of Motion
Kinetic energy is the energy associated with the motion of particles. At higher temperatures, particles have more kinetic energy, moving faster and more vigorously. This increased kinetic energy can overcome intermolecular forces, leading to a change in state.
For instance, as a solid is heated, its particles gain kinetic energy, eventually overcoming the strong intermolecular forces holding them in a fixed structure. The solid melts, transitioning to the liquid state. Further heating increases kinetic energy even more, leading to the liquid boiling and transforming into a gas.
Why Liquids Adapt Their Shape: A Detailed Explanation
The lack of a definite shape in liquids stems directly from the balance between intermolecular forces and kinetic energy. The intermolecular forces are strong enough to keep the molecules relatively close together, maintaining a relatively constant volume. However, they are not strong enough to hold the molecules in a rigid, fixed arrangement.
The particles in a liquid possess enough kinetic energy to move and slide past each other, allowing the liquid to conform to the shape of its container. This movement is not completely random; it is influenced by the intermolecular forces, which prevent the liquid from completely dispersing like a gas.
Imagine a swarm of bees in a hive. The bees are relatively close together, but they can move and rearrange themselves within the confines of the hive. Similarly, the molecules in a liquid are close together, but their ability to move and slide past one another allows them to adjust their arrangement to fit the container's shape.
Factors Affecting Liquid Shape and Volume: Temperature and Pressure
While the volume of a liquid generally remains constant under normal conditions, temperature and pressure can influence both its shape and volume.
Temperature's Role
Increasing the temperature of a liquid increases the kinetic energy of its particles, causing them to move more rapidly and spread out slightly. This results in a small increase in volume, a phenomenon known as thermal expansion. However, the change in shape is still dictated by the container.
Pressure's Influence
Applying pressure to a liquid compresses its molecules slightly, reducing its volume. However, liquids are much less compressible than gases because their particles are already relatively close together. The shape, again, remains dependent on the containing vessel.
Exceptions and Complexities: Surfactants and Liquid Crystals
While the general rule is that liquids lack a definite shape, there are some exceptions and complexities worth noting:
Surfactants: Modifying Surface Tension
Surfactants (surface-active agents) are substances that reduce the surface tension of a liquid. Surface tension is the tendency of liquid surfaces to minimize their area, causing liquids to form droplets and resist changes in shape. Surfactants disrupt the surface tension, allowing liquids to spread more easily and potentially exhibit altered shapes. This is observed in detergents, which allow water to wet surfaces more effectively.
Liquid Crystals: Ordered Disorder
Liquid crystals are a fascinating state of matter that bridges the gap between liquids and solids. They exhibit some properties of solids, such as a degree of ordering, while retaining some fluidity characteristic of liquids. They can respond to electric and magnetic fields, changing their shape and optical properties accordingly, making them useful in LCD screens. Their behavior exemplifies the complex relationship between molecular arrangement and macroscopic properties.
Conclusion: The Adaptable Nature of Liquids
In conclusion, liquids do not possess a definite shape. Their characteristic ability to flow and conform to the shape of their container arises from the balance between intermolecular forces and the kinetic energy of their constituent particles. While temperature and pressure can slightly influence their volume, the lack of a fixed structure remains a defining feature. Understanding the microscopic behavior of liquid molecules allows us to appreciate their adaptability and the fascinating properties they exhibit. The exceptions and complexities, such as the influence of surfactants and the unique properties of liquid crystals, further highlight the rich and nuanced nature of the liquid state of matter.
Latest Posts
Latest Posts
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Liquids Have A Definite Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
