Do Nonmetals Have High Ionization Energy
Muz Play
Mar 24, 2025 · 6 min read
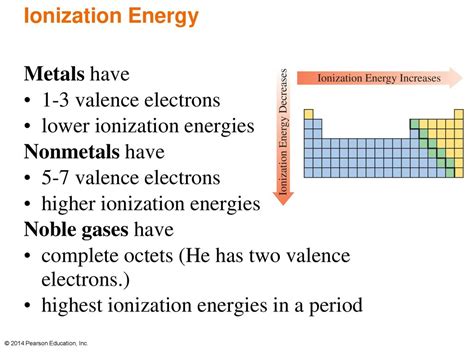
Table of Contents
Do Nonmetals Have High Ionization Energy? Exploring the Periodic Trends
Ionization energy, the minimum energy required to remove an electron from a neutral gaseous atom, is a fundamental property reflecting an element's electronegativity and reactivity. Understanding ionization energy trends across the periodic table is crucial for predicting chemical behavior. This article delves into the relationship between nonmetals and their characteristically high ionization energies, exploring the underlying reasons and exceptions.
The Fundamental Nature of Ionization Energy
Before focusing on nonmetals, let's establish a firm grasp of ionization energy itself. The process of ionization involves overcoming the electrostatic attraction between the negatively charged electron and the positively charged nucleus. The stronger this attraction, the higher the ionization energy. Several factors influence this attraction:
Nuclear Charge:
A higher nuclear charge (more protons) exerts a stronger pull on the electrons, increasing ionization energy. As you move across a period (left to right) in the periodic table, the nuclear charge increases, leading to a general increase in ionization energy.
Atomic Radius:
Electrons closer to the nucleus experience a stronger attraction. Smaller atomic radii translate to higher ionization energies. As you move across a period, atomic radius generally decreases, contributing to the increase in ionization energy. Down a group, atomic radius increases, leading to a decrease in ionization energy.
Shielding Effect:
Inner electrons shield outer electrons from the full positive charge of the nucleus, reducing the effective nuclear charge experienced by the outer electrons. This shielding effect is more pronounced in elements with more electron shells. Therefore, elements with more electron shells generally have lower ionization energies.
Electron Configuration:
Electrons in filled or half-filled subshells are more stable than those in partially filled subshells. Removing an electron from a stable configuration requires more energy, resulting in higher ionization energy. This effect can cause slight deviations from the general trends.
Nonmetals and Their High Ionization Energies
Nonmetals, located on the right-hand side of the periodic table, generally exhibit high ionization energies. This characteristic is directly linked to their electronic structure and the factors discussed above.
Stronger Nuclear Attraction:
Nonmetals have a relatively high number of protons compared to the number of electron shells. This leads to a stronger effective nuclear charge, resulting in a tighter hold on their valence electrons. The valence electrons are the outermost electrons and are involved in chemical bonding. The strong attraction makes it difficult to remove these electrons, hence the high ionization energy.
Smaller Atomic Radii:
Nonmetals tend to have smaller atomic radii compared to metals. This smaller size translates to a shorter distance between the nucleus and the valence electrons, leading to stronger electrostatic attraction and higher ionization energy. The compact nature of their electron clouds contributes significantly to their high ionization energy values.
Filled or Partially Filled Subshells:
Many nonmetals have either completely filled or half-filled subshells, which are relatively stable electronic configurations. Removing an electron from these stable arrangements requires a substantial amount of energy, resulting in a high ionization energy. This enhanced stability contributes to the nonmetals' characteristically high ionization energy.
Exceptions and Irregularities
While nonmetals generally exhibit high ionization energies, some exceptions exist due to the subtle interplay of the factors discussed earlier:
The Anomalous Behavior of Oxygen and Nitrogen:
Oxygen and nitrogen demonstrate an anomaly in the ionization energy trend across the second period. Although oxygen comes after nitrogen, it has a slightly lower first ionization energy. This deviation is attributed to the electronic configuration. Nitrogen has a half-filled p subshell (p³ configuration), which is relatively stable. Removing an electron from this stable configuration requires more energy than removing an electron from oxygen, which has a p⁴ configuration (one electron pairing in the p subshell). This subtle electronic configuration difference explains this counter-intuitive observation.
The Influence of Electron-Electron Repulsion:
While the effective nuclear charge significantly impacts ionization energy, electron-electron repulsion also plays a role. In some cases, the repulsion between electrons can slightly outweigh the increased nuclear attraction, leading to a slightly lower ionization energy than expected. This effect is particularly noticeable in larger atoms where electron-electron interactions become more significant.
The Role of Subshell Penetration:
The degree of penetration of electrons in different subshells influences ionization energy. Electrons in s subshells penetrate closer to the nucleus than electrons in p subshells. This penetration effect can influence the energy required to remove an electron, sometimes causing small deviations from the general trends.
Comparing Ionization Energies: Metals vs. Nonmetals
The contrast between metals and nonmetals in terms of ionization energy is stark. Metals, located on the left-hand side of the periodic table, generally have low ionization energies. This is because they have fewer protons relative to their electron shells, leading to weaker nuclear attraction. Their larger atomic radii further contribute to the lower ionization energies. The ease of removing electrons from metals accounts for their metallic properties such as conductivity and malleability. The difference between the ionization energies of metals and nonmetals directly impacts their chemical reactivity and the types of bonds they form (ionic versus covalent).
Applications and Significance of Understanding Ionization Energy
Understanding ionization energy trends, particularly the high ionization energies of nonmetals, has broad applications across various scientific fields:
Predicting Chemical Reactivity:
Ionization energy provides valuable insights into an element's reactivity. Elements with high ionization energies are less likely to lose electrons and tend to gain electrons to achieve a stable electron configuration, forming anions. This behavior is characteristic of nonmetals and is crucial in understanding their role in forming covalent compounds.
Understanding Bonding:
The difference in ionization energies between metals and nonmetals explains the formation of ionic bonds. Metals, with low ionization energies, readily lose electrons, while nonmetals, with high ionization energies, readily gain electrons. This transfer of electrons leads to the formation of ionic compounds. The ionization energy difference also influences the properties of covalent bonds formed between nonmetals.
Spectroscopy and Atomic Physics:
The precise measurement of ionization energies is essential in various spectroscopic techniques used to analyze the composition of materials. Ionization energies are fundamental data used in understanding atomic structure and electron behavior.
Materials Science and Engineering:
Ionization energy is a crucial parameter in materials science and engineering for designing materials with specific properties. For instance, understanding the ionization energies of elements in semiconductors is vital in developing electronic devices.
Chemical Industry and Applications:
Many chemical processes rely on the knowledge of ionization energies. For example, understanding the ionization energies of gases helps in designing efficient plasma processes and controlling chemical reactions.
Conclusion
In summary, nonmetals generally exhibit high ionization energies due to their strong nuclear attraction, smaller atomic radii, and often stable electron configurations. However, exceptions and irregularities exist, emphasizing the complex interplay of electronic structure and quantum mechanical effects. Understanding ionization energy trends is crucial for predicting chemical behavior, designing materials with specific properties, and advancing various scientific and technological applications. The significant difference in ionization energies between metals and nonmetals underpins the diverse chemical properties and bonding behavior observed across the periodic table, shaping the world around us.
Latest Posts
Latest Posts
-
When Elements Combine To Form Compounds
Mar 25, 2025
-
Describe The Sampling Distribution Of P Hat
Mar 25, 2025
-
How To Determine State Of Matter In Chemical Equation
Mar 25, 2025
-
Resistors In Series And Parallel Calculator
Mar 25, 2025
-
Where Are Breathing Control Centers Located
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Do Nonmetals Have High Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
