Does A Liquid Have A Definite Shape
Muz Play
Mar 24, 2025 · 6 min read
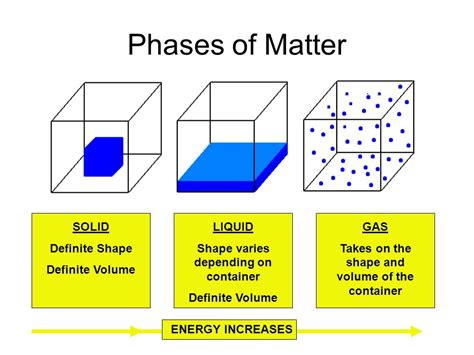
Table of Contents
Does a Liquid Have a Definite Shape? Exploring the Properties of Liquids
The question of whether a liquid has a definite shape is a fundamental concept in understanding the states of matter. Unlike solids, which possess both definite shape and volume, liquids exhibit a fascinating interplay between form and containment. This article delves into the microscopic behavior of liquids, explores the forces that govern their shape, and examines various scenarios that highlight the fluid nature of liquids. We’ll also discuss the relationship between shape and other liquid properties like viscosity and surface tension.
Understanding the States of Matter: A Molecular Perspective
To understand why liquids lack a definite shape, we must first consider the behavior of molecules within different states of matter. In solids, molecules are tightly packed in a rigid, ordered structure, held together by strong intermolecular forces. This rigid structure dictates the solid's definite shape and volume.
Solids: A World of Order
Imagine soldiers standing rigidly in formation. This is analogous to the arrangement of molecules in a solid. Their fixed positions resist any external force attempting to alter their shape or volume. This is why a solid maintains its form, regardless of the container it occupies.
Liquids: A Dynamic Equilibrium
In contrast to solids, liquid molecules are less tightly packed and possess greater freedom of movement. They are still subject to intermolecular forces, but these forces are weaker and less restrictive than in solids. Think of a bustling marketplace – people are moving around, interacting, but not in a rigid formation. This constant motion and less-ordered arrangement allow liquids to adapt their shape to their container.
Gases: The Ultimate Freedom
Gases represent the ultimate state of disorder. Molecules in a gas are widely dispersed and move freely with minimal interaction. They lack both a definite shape and volume, readily expanding to fill any available space.
Why Liquids Conform to Their Containers: The Role of Intermolecular Forces
The lack of a definite shape in liquids stems directly from the nature of intermolecular forces. While these forces are weaker than in solids, they are still significant enough to prevent liquids from completely dispersing like gases. Instead, they dictate the overall behavior and properties of the liquid.
Intermolecular Forces: The Glue that Holds Liquids Together
Several types of intermolecular forces influence liquid behavior, including:
- Van der Waals forces: These weak, temporary attractions arise from fluctuations in electron distribution around molecules. They are present in all molecules but are particularly weak in non-polar molecules.
- Dipole-dipole interactions: These occur between polar molecules with permanent dipoles (unequal charge distribution). The positive end of one molecule attracts the negative end of another.
- Hydrogen bonding: A special type of dipole-dipole interaction involving hydrogen atoms bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine. Hydrogen bonds are stronger than typical dipole-dipole interactions.
These forces, while weaker than covalent or ionic bonds within molecules, are strong enough to hold liquid molecules relatively close together. This proximity explains the definite volume of liquids – they resist compression. However, the relative weakness and dynamic nature of these forces allow the molecules to flow and rearrange, explaining why liquids adapt to the shape of their container.
Surface Tension: The Skin of a Liquid
Surface tension is a critical property of liquids closely related to their shape. It's the tendency of liquid surfaces to minimize their area, behaving like a stretched elastic membrane. This minimization of surface area arises from the imbalance of intermolecular forces at the liquid's surface. Molecules inside the bulk of the liquid experience attractive forces from all directions. However, surface molecules experience a net inward force, pulling them towards the interior. This inward pull causes the surface to contract, minimizing its area.
Surface tension manifests in various ways:
- Water droplets forming spheres: A sphere has the minimum surface area for a given volume. Surface tension minimizes the surface area of water droplets, making them nearly spherical.
- Insects walking on water: The surface tension is strong enough to support the weight of small insects.
- Capillary action: The ability of liquids to climb up narrow tubes against gravity is partially due to surface tension and adhesive forces between the liquid and the tube's walls.
Viscosity: Resistance to Flow
Viscosity is another important property that influences how a liquid behaves and its apparent shape. Viscosity measures a liquid's resistance to flow. High-viscosity liquids like honey flow slowly, while low-viscosity liquids like water flow readily.
The viscosity of a liquid depends on several factors, including:
- Intermolecular forces: Stronger intermolecular forces lead to higher viscosity because molecules are more resistant to movement past each other.
- Temperature: Increased temperature reduces viscosity because molecules gain kinetic energy, overcoming intermolecular forces more easily.
- Molecular shape and size: Larger or more complex molecules generally result in higher viscosity due to increased intermolecular interactions.
Viscosity affects how quickly a liquid conforms to the shape of its container. High-viscosity liquids take longer to settle into the container's shape compared to low-viscosity liquids.
Exploring Different Scenarios: Liquid Shape in Action
Let's consider a few scenarios to further illustrate the lack of definite shape in liquids:
Scenario 1: Pouring Water into Different Containers
Pouring water into a tall, narrow glass results in a column of water. Pouring the same amount of water into a wide, shallow dish results in a thin layer. In both cases, the water takes on the shape of the container, demonstrating the lack of a definite shape.
Scenario 2: Observing Water in a Spherical Container
If you place water in a spherical container, the water will, indeed, take on a nearly spherical shape. However, this is a consequence of the surface tension minimizing surface area, not an inherent property of the water molecules themselves, emphasizing again the absence of a definite shape.
Scenario 3: Considering Liquids Under Microgravity
In the microgravity environment of space, surface tension dominates the shape of liquids. Water forms perfect spheres because there's no gravitational force to pull it downwards. This again highlights the lack of inherent shape; external factors are dictating the observable shape.
Conclusion: A Shape Defined by its Surroundings
In conclusion, a liquid does not possess a definite shape. Its molecules are not rigidly fixed like in a solid, and their movement and interactions, governed by intermolecular forces, allow them to conform to the shape of their container. While surface tension plays a role in shaping the liquid’s surface, it is the container that ultimately dictates the overall shape. The properties of viscosity and the influence of external factors such as gravity further underscore the fluid nature of liquids and their remarkable ability to adapt. The shape of a liquid is not an intrinsic characteristic but rather a response to its environment.
Latest Posts
Latest Posts
-
Soave Redlich Kwong Equation Of State
Mar 26, 2025
-
Words That Start With J In Biology
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Does A Liquid Have A Definite Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
