Soave Redlich Kwong Equation Of State
Muz Play
Mar 26, 2025 · 6 min read
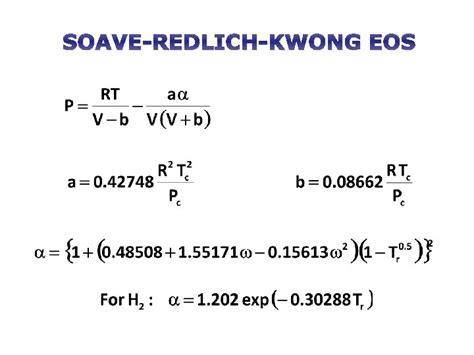
Table of Contents
Soave-Redlich-Kwong Equation of State: A Comprehensive Guide
The Soave-Redlich-Kwong (SRK) equation of state is a cubic equation that's widely used in chemical engineering to model the PVT (pressure-volume-temperature) behavior of fluids, particularly in the petroleum and natural gas industries. It's an improvement upon the original Redlich-Kwong equation of state, offering enhanced accuracy, especially for predicting the properties of liquids. This article will delve into the SRK equation, its derivation, applications, advantages, limitations, and comparisons with other equations of state.
Understanding the SRK Equation
The SRK equation is expressed as:
P = (RT)/(V-b) - aα/(V(V+b))
Where:
- P represents pressure
- V represents molar volume
- R is the ideal gas constant
- T is the absolute temperature
- a and b are parameters specific to each substance, representing the attractive and repulsive forces between molecules, respectively.
- α is a temperature-dependent function that accounts for the deviation from the original Redlich-Kwong equation, improving accuracy at higher temperatures and pressures.
The Significance of the Temperature-Dependent Function (α)
The original Redlich-Kwong equation struggled to accurately predict the behavior of fluids near the critical point and in the liquid phase. Soave's modification introduced the temperature-dependent function α, significantly improving the equation's accuracy for a wider range of temperatures and pressures. This function is typically calculated using a correlation based on the acentric factor (ω) of the substance:
α = [1 + m(1 - T<sub>r</sub><sup>0.5</sup>)]<sup>2</sup>
Where:
- m = 0.48 + 1.574ω - 0.176ω<sup>2</sup>
- T<sub>r</sub> = T/T<sub>c</sub> is the reduced temperature (T is the absolute temperature, and T<sub>c</sub> is the critical temperature).
- ω is the acentric factor, an empirical parameter that characterizes the deviation of a substance's properties from those of an ideal gas.
Derivation and Theoretical Background
The SRK equation, like other cubic equations of state, is an empirical equation derived from modifications to the ideal gas law. The ideal gas law, while simple, fails to accurately describe the behavior of real fluids, especially at high pressures and low temperatures. Real fluids exhibit intermolecular forces (attractive and repulsive) that are not accounted for in the ideal gas law.
The Redlich-Kwong equation was a significant step forward, introducing terms to account for these intermolecular forces. However, its performance, particularly for liquids, was limited. Soave refined the equation by adding the temperature-dependent function α, which significantly improved the accuracy of the equation, especially in representing the liquid phase. The exact derivation is complex and involves statistical mechanics principles and approximations, but the core idea is to incorporate a more realistic representation of intermolecular forces.
Application of the SRK Equation
The SRK equation has a broad range of applications in various chemical engineering processes:
1. Phase Equilibrium Calculations:**
The SRK equation is frequently used to calculate vapor-liquid equilibrium (VLE) for mixtures of hydrocarbons and other components. This is crucial in designing and optimizing separation processes like distillation, absorption, and extraction.
2. PVT Property Predictions:**
It provides accurate estimations of pressure, volume, and temperature for pure substances and mixtures over a wide range of conditions. This is essential for designing and sizing process equipment, such as compressors, pumps, and heat exchangers.
3. Thermodynamic Property Calculations:**
Derived thermodynamic properties, such as enthalpy, entropy, and fugacity, can be calculated using the SRK equation. These properties are necessary for performing energy balances and evaluating the feasibility of various process options.
4. Process Simulation and Optimization:**
The SRK equation is implemented in many process simulation software packages (like Aspen Plus, ProMax, etc.) to model and optimize complex chemical processes.
Advantages of the SRK Equation
- Relatively Simple and Efficient: Compared to more complex equations of state, the SRK equation is computationally less demanding, making it suitable for use in large-scale simulations.
- Wide Applicability: It can be applied to a broad range of substances, including hydrocarbons, refrigerants, and other chemicals.
- Improved Accuracy: The inclusion of the temperature-dependent function (α) significantly improves its accuracy compared to the original Redlich-Kwong equation, particularly for liquid phases.
- Widely Available: The SRK equation is implemented in many commercial and open-source process simulation software packages.
Limitations of the SRK Equation
Despite its advantages, the SRK equation does have some limitations:
- Accuracy Limitations: While an improvement over the Redlich-Kwong equation, the SRK equation's accuracy is still limited, especially for highly polar substances and substances with strong hydrogen bonding. For these substances, more sophisticated equations of state may be necessary.
- Critical Region Accuracy: Even with the α function, the accuracy of the SRK equation near the critical point is still not perfect. The equation tends to deviate from experimental data in this region.
- Mixing Rules: The accuracy of the SRK equation also depends on the choice of mixing rules used for mixtures. Different mixing rules can yield different results, and the best choice may depend on the specific application.
Comparison with Other Equations of State
The SRK equation is just one among many equations of state used in chemical engineering. It is often compared to other cubic equations of state, such as the Peng-Robinson (PR) equation.
The PR equation also incorporates a temperature-dependent function, but it uses a different form of the function, resulting in improved accuracy for certain substances, particularly those with stronger intermolecular forces. Both SRK and PR equations are frequently used, and the choice between them often depends on the specific application and the substances involved. Other, more complex equations of state, such as the SAFT (Statistical Associating Fluid Theory) equations, offer better accuracy but are significantly more computationally intensive.
Improving Accuracy and Handling Complex Mixtures
While the SRK equation offers a balance between accuracy and computational efficiency, several strategies can further improve its accuracy and broaden its applicability. These include:
- Modified α functions: Researchers have developed various modifications to the original α function to improve accuracy for specific substances or temperature ranges. These modifications often involve adding additional parameters or adjusting the existing parameters based on experimental data.
- Improved mixing rules: The choice of mixing rule significantly impacts the accuracy of the SRK equation when applied to mixtures. More sophisticated mixing rules, such as those based on activity coefficients or the composition-dependent parameters, can significantly improve the accuracy of phase equilibrium predictions.
- Combining with other models: The SRK equation can be combined with other thermodynamic models to improve accuracy for specific regions or properties. For instance, it can be coupled with activity coefficient models to enhance the prediction of liquid-phase properties in highly non-ideal mixtures.
Conclusion
The Soave-Redlich-Kwong equation of state remains a valuable tool in chemical engineering due to its balance of accuracy and computational efficiency. While it has limitations, particularly with highly polar substances and near critical conditions, it provides a reasonable approximation for many industrial applications. Understanding its strengths, weaknesses, and modifications allows engineers to choose the most appropriate equation of state for their specific needs, ensuring accurate and reliable predictions in process design, optimization, and simulation. The ongoing research and development in refining the SRK equation and incorporating it with other models ensure its continued relevance in the chemical and process industries.
Latest Posts
Latest Posts
-
Record The Adjusting Entry For Uncollectible Accounts
Mar 29, 2025
-
What Is Group 1a On The Periodic Table
Mar 29, 2025
-
Chapter 1 Biology The Study Of Life
Mar 29, 2025
-
Finding Critical Points Of Multivariable Functions
Mar 29, 2025
-
Why Is Frozen Water Less Dense Than Liquid Water
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Soave Redlich Kwong Equation Of State . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
