Why Is Frozen Water Less Dense Than Liquid Water
Muz Play
Mar 29, 2025 · 5 min read
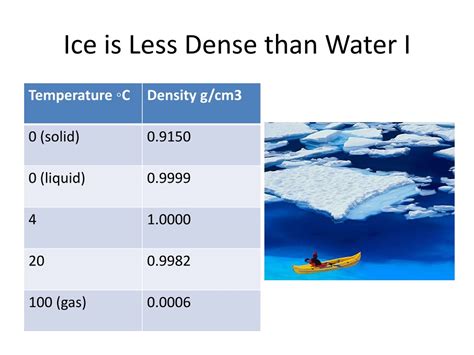
Table of Contents
Why Is Frozen Water Less Dense Than Liquid Water? An In-Depth Look at a Unique Property
Water, the elixir of life, exhibits a peculiar anomaly that sets it apart from most other substances: its solid form, ice, is less dense than its liquid form. This seemingly simple fact has profound implications for life on Earth, influencing everything from the habitability of our planet to the intricate workings of aquatic ecosystems. Understanding why ice floats is crucial to grasping the fundamental properties of water and its vital role in shaping our world.
The Dance of Hydrogen Bonds: The Key to Water's Anomaly
The unusual behavior of water stems from the nature of its molecular structure and the powerful intermolecular forces that govern its interactions. Water molecules (H₂O) are composed of two hydrogen atoms covalently bonded to a single oxygen atom. Oxygen, being more electronegative, attracts the shared electrons more strongly, creating a polar molecule with a slightly negative charge near the oxygen atom and slightly positive charges near the hydrogen atoms.
This polarity allows water molecules to engage in hydrogen bonding, a special type of intermolecular attraction. The slightly positive hydrogen atom of one water molecule is attracted to the slightly negative oxygen atom of a neighboring molecule. These hydrogen bonds are relatively weak compared to covalent bonds, but they are strong enough to significantly influence the properties of water.
In liquid water, hydrogen bonds are constantly forming and breaking as the molecules move around. This dynamic network is responsible for water's high surface tension, specific heat capacity, and boiling point. However, the arrangement of these bonds is relatively disordered, allowing for a higher density of molecules.
The Crystalline Structure of Ice: A Story of Space and Stability
When water freezes, the hydrogen bonds become more ordered and stable. The molecules arrange themselves into a hexagonal crystalline lattice, a precise three-dimensional structure with relatively large spaces between the molecules. This structure is maximized for hydrogen bond stability, creating a less densely packed arrangement than in liquid water.
Think of it like arranging oranges in a crate. You can pack them more tightly together in a random pile (liquid water), but if you arrange them in a structured pattern (ice), you'll have more empty space. This increased spacing between water molecules in the ice crystal lattice is the primary reason why ice is less dense than liquid water.
The Implications of this Lower Density: A Crucial Factor for Life
This seemingly simple difference in density has far-reaching consequences for life on Earth. Because ice floats, it forms an insulating layer on the surface of lakes and oceans during winter. This layer prevents the water beneath from freezing solid, allowing aquatic life to survive even in extremely cold temperatures. If ice were denser than water, it would sink, leading to the complete freezing of water bodies from the bottom up, making aquatic life virtually impossible.
The role of ice in moderating global temperatures is also significant. The high albedo (reflectivity) of ice and snow contributes to the Earth's overall energy balance, influencing climate patterns and preventing drastic temperature fluctuations.
Examining the Density Difference: A Closer Look at the Numbers
The density of ice at 0°C is approximately 0.917 g/cm³, while the density of liquid water at the same temperature is approximately 0.9998 g/cm³. This seemingly small difference of about 9% has enormous consequences, as we've discussed.
This density difference is also affected by pressure. As pressure increases, the density of ice increases, and at extremely high pressures, ice can become denser than liquid water. However, these conditions are not typically found in natural environments on Earth.
Beyond the Basics: Exploring Other Factors Affecting Density
While hydrogen bonding and the crystalline structure are the primary reasons for the lower density of ice, other factors play a minor role:
- Zero-point energy: Even at absolute zero, molecules possess some residual vibrational energy, which contributes to the spacing between molecules.
- Thermal expansion: Although less significant than the effect of the crystalline structure, thermal expansion also slightly affects the density of both liquid water and ice.
- Isotopic composition: The isotopic composition of water (the relative abundance of different isotopes of hydrogen and oxygen) can subtly affect its density, though this effect is generally minor.
The Scientific Study of Water: An Ongoing Exploration
The unique properties of water, including its anomalous density behavior, have been the subject of extensive scientific investigation for centuries. Scientists continue to explore the intricacies of water's behavior through advanced techniques like X-ray diffraction, neutron scattering, and molecular dynamics simulations, providing a deeper understanding of the fundamental forces that govern this remarkable substance.
Conclusion: A Simple Fact with Profound Consequences
The fact that ice is less dense than liquid water is far from trivial. This unique property is a fundamental aspect of water's behavior, driving its role in shaping the Earth's climate, supporting aquatic ecosystems, and making life as we know it possible. The seemingly simple dance of hydrogen bonds reveals a complexity that continues to fascinate and inspire scientific inquiry, reminding us of the profound impact of seemingly simple scientific principles on the world around us. Understanding this anomaly highlights the interconnectedness of various scientific fields and underscores the significance of studying the seemingly simple to unravel the complexities of our world. From the microcosm of molecular interactions to the macrocosm of global climate patterns, the lower density of ice compared to liquid water plays a pivotal role in shaping the planet we inhabit.
Latest Posts
Latest Posts
-
What Does Mic Stand For In Microbiology
Mar 31, 2025
-
Multiplying A Polynomial By A Monomial
Mar 31, 2025
-
How Is Fermentation Different From Cellular Respiration
Mar 31, 2025
-
Unit Of Rate Constant For Second Order Reaction
Mar 31, 2025
-
Where On The Periodic Table Are The Nonmetals Located
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Why Is Frozen Water Less Dense Than Liquid Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
