What Does Mic Stand For In Microbiology
Muz Play
Mar 31, 2025 · 6 min read
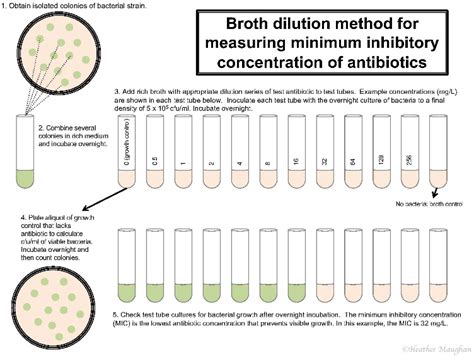
Table of Contents
What Does MIC Stand For in Microbiology? Understanding Minimum Inhibitory Concentration
In the intricate world of microbiology, understanding the dynamics of antimicrobial agents and their effects on microbial growth is paramount. One crucial term frequently encountered in this field is MIC, which stands for Minimum Inhibitory Concentration. This article delves deep into the meaning, significance, and applications of MIC, exploring its role in guiding treatment strategies, antibiotic susceptibility testing, and the overall fight against infectious diseases.
Defining Minimum Inhibitory Concentration (MIC)
The Minimum Inhibitory Concentration (MIC) is defined as the lowest concentration of an antimicrobial agent (e.g., antibiotic, antifungal, antiviral) that prevents visible growth of a specific microorganism after a defined incubation period. It's a critical parameter that helps determine the efficacy of an antimicrobial against a particular pathogen. Unlike the Minimum Bactericidal Concentration (MBC), which assesses the lowest concentration needed to kill the microorganism, the MIC focuses solely on inhibition of growth. This means that even though growth is visibly inhibited at the MIC, some microorganisms might still survive.
The Importance of the "Visible Growth" Criterion
The "visible growth" aspect is crucial in MIC determination. It typically involves visually inspecting the growth media for turbidity (cloudiness), indicating microbial proliferation. The absence of turbidity suggests growth inhibition. More sophisticated methods, including spectrophotometry, can quantify microbial growth more precisely. However, the fundamental principle remains the same: identifying the lowest concentration at which growth is demonstrably prevented.
How MIC is Determined: Methods and Techniques
Several methods are employed to determine the MIC of an antimicrobial agent against a specific microorganism. These methods generally involve exposing the microorganism to a range of antimicrobial concentrations and assessing its growth. The most common techniques include:
1. Broth Microdilution Method: A Standard Approach
The broth microdilution method is widely considered the gold standard for MIC determination. It involves preparing serial dilutions of the antimicrobial agent in a suitable broth medium. A standardized inoculum of the test microorganism is then added to each dilution. After incubation, the lowest concentration exhibiting no visible growth is recorded as the MIC. This method allows for high throughput and accurate results.
2. Agar Dilution Method: A Versatile Alternative
The agar dilution method involves incorporating different concentrations of the antimicrobial agent directly into the agar medium before pouring the plates. The test organism is then inoculated onto the plates, and the MIC is determined by observing the lowest concentration that inhibits visible growth after incubation. This method is particularly useful when testing many different isolates against a single antimicrobial agent.
3. Disk Diffusion Method (Kirby-Bauer Test): A Qualitative Approach
While not providing a precise MIC value, the disk diffusion method, also known as the Kirby-Bauer test, offers a qualitative assessment of antimicrobial susceptibility. Antimicrobial-impregnated disks are placed on an agar plate inoculated with the test microorganism. The zone of inhibition (the area around the disk where microbial growth is inhibited) is measured and compared to standardized tables to categorize the organism as susceptible, intermediate, or resistant. While less precise than broth or agar dilution, it's a rapid and widely used method for initial screening.
4. Etest: A Gradient Diffusion Technique
The Etest utilizes a plastic strip containing a gradient of antimicrobial concentrations. The strip is placed on an inoculated agar plate, and after incubation, the MIC is determined by the intersection of the elliptical zone of inhibition with the strip's concentration scale. This method provides a more continuous range of concentrations compared to disk diffusion, offering a more precise MIC estimate.
Factors Influencing MIC Values
Several factors can influence the MIC value obtained for a given microorganism and antimicrobial agent:
-
Inoculum size: The initial number of microorganisms in the test affects the MIC. A higher inoculum may require a higher antimicrobial concentration to inhibit growth.
-
Growth medium composition: The nutrients and other components in the growth medium can impact microbial growth and therefore the MIC.
-
Incubation conditions: Temperature, time, and atmosphere during incubation can influence the MIC.
-
Antimicrobial properties: The inherent properties of the antimicrobial agent, such as its stability, solubility, and mechanism of action, affect its inhibitory capacity.
-
Bacterial characteristics: The inherent properties of the microorganism, including its species, strain, and genetic makeup, play a crucial role in determining its susceptibility to the antimicrobial agent. For instance, the presence of efflux pumps, which expel antimicrobial agents from the bacterial cell, can significantly impact MIC values.
The Significance of MIC in Clinical Practice
MIC values are crucial in clinical practice for several reasons:
-
Guiding antimicrobial therapy: MIC values help clinicians choose the most appropriate antimicrobial agent and dose for treating a particular infection. An antimicrobial with a lower MIC against the infecting organism is generally preferred.
-
Monitoring antimicrobial resistance: Tracking changes in MIC values over time can help monitor the development of antimicrobial resistance in bacterial populations. Increases in MIC values suggest a decline in antimicrobial susceptibility.
-
Evaluating new antimicrobial agents: MIC determination is crucial in evaluating the efficacy of newly developed antimicrobial agents.
-
Epidemiology and infection control: MIC data can be used to track the prevalence of antimicrobial resistance in hospitals and other healthcare settings, guiding infection control strategies.
MIC and Antimicrobial Resistance
The development of antimicrobial resistance is a major global health concern. Resistance arises when microorganisms develop mechanisms to evade or counteract the effects of antimicrobial agents. This often leads to increased MIC values. Understanding MICs is critical for combating resistance by:
-
Identifying resistant strains: High MIC values indicate resistance, enabling clinicians to select appropriate alternative therapies.
-
Developing new antimicrobial agents: Research and development of new antimicrobial agents often involve determining MIC values against various resistant strains.
-
Implementing infection control measures: Understanding the MICs of prevalent pathogens can aid in designing infection control strategies to minimize the spread of resistant organisms.
-
Promoting judicious antimicrobial use: Understanding the importance of MIC and its implications in antimicrobial stewardship helps clinicians and healthcare professionals to prescribe antibiotics only when necessary.
Beyond MIC: Exploring MBC and Other Parameters
While MIC is a primary indicator of antimicrobial effectiveness, other parameters, such as the Minimum Bactericidal Concentration (MBC), provide additional information. The MBC is the lowest concentration of an antimicrobial agent that kills 99.9% of the initial inoculum. This distinction between inhibiting growth (MIC) and killing the bacteria (MBC) is crucial in understanding the antimicrobial’s action. A low MBC indicates a bactericidal effect, while a high MBC or no significant difference from the MIC suggests a bacteriostatic effect.
Other parameters used in conjunction with MIC include:
-
Time-kill curves: These curves plot the change in viable bacterial count over time at different antimicrobial concentrations, providing a detailed picture of the antimicrobial's effect.
-
Post-antibiotic effect (PAE): This refers to the continued suppression of bacterial growth after exposure to an antimicrobial agent has ceased. PAE is an important factor in determining the dosing frequency of antimicrobials.
-
Pharmacokinetic/pharmacodynamic (PK/PD) indices: These parameters integrate the pharmacokinetic (drug concentration in the body) and pharmacodynamic (drug effect on the microorganism) properties of an antimicrobial agent to predict its clinical efficacy.
Conclusion: MIC—A Cornerstone of Antimicrobial Susceptibility Testing
The Minimum Inhibitory Concentration (MIC) serves as a cornerstone in antimicrobial susceptibility testing, guiding treatment decisions, monitoring antimicrobial resistance, and advancing the understanding of antimicrobial agents' effectiveness. The methods used for determining MIC—broth microdilution, agar dilution, disk diffusion, and Etest—provide varying levels of precision and are selected based on the specific needs of the testing scenario. Furthermore, understanding the factors influencing MIC values and integrating them with other parameters like MBC and PK/PD indices is critical for effectively combating infectious diseases and the global challenge of antimicrobial resistance. The continued development of accurate, rapid, and accessible MIC determination methods is essential for optimizing antimicrobial therapy and safeguarding global health.
Latest Posts
Latest Posts
-
Is The Hydrolysis Of Atp Endergonic Or Exergonic
Apr 02, 2025
-
Choose The Component Parts Of An Amino Acid
Apr 02, 2025
-
Label Each Type Of Intercellular Junction
Apr 02, 2025
-
A Person Throws A Marble Straight Up
Apr 02, 2025
-
Label These Structures Of The Upper Respiratory System
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Does Mic Stand For In Microbiology . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
