Unit Of Rate Constant For Second Order Reaction
Muz Play
Mar 31, 2025 · 6 min read
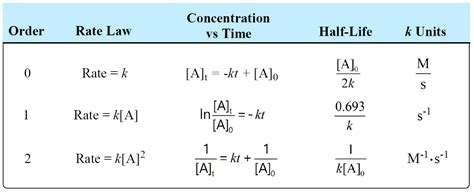
Table of Contents
Understanding the Unit of Rate Constant for Second Order Reactions
The rate constant, often denoted as k, is a crucial parameter in chemical kinetics. It quantifies the rate of a reaction at a given temperature, providing insight into reaction speed and mechanism. For second-order reactions, the unit of the rate constant differs from that of first-order or zero-order reactions, reflecting the dependence of the reaction rate on the concentration of reactants. This article delves deep into understanding the unit of the rate constant for second-order reactions, exploring its derivation, variations, and significance in different scenarios.
Defining Second-Order Reactions
Before diving into the units of the rate constant, let's solidify our understanding of what constitutes a second-order reaction. A second-order reaction is a chemical reaction whose rate depends on the concentration of one reactant raised to the second power or on the concentration of two reactants each raised to the first power. This means the reaction rate is directly proportional to the square of the concentration of a single reactant or the product of the concentrations of two reactants.
There are two main types of second-order reactions:
1. Second-Order Reaction with One Reactant (Type A):
The rate law for this type takes the form:
Rate = k [A]²
where:
- Rate: represents the speed of the reaction (often expressed as change in concentration per unit time, e.g., mol L⁻¹ s⁻¹).
- k: is the rate constant (the value we're focusing on).
- [A]: represents the concentration of reactant A (typically in mol L⁻¹).
2. Second-Order Reaction with Two Reactants (Type B):
The rate law here is:
Rate = k [A][B]
where:
- Rate: remains the reaction speed (mol L⁻¹ s⁻¹).
- k: is again the rate constant.
- [A] and [B]: represent the concentrations of reactants A and B (both in mol L⁻¹).
Deriving the Unit of the Rate Constant (k)
To determine the units of k, we need to rearrange the rate law equations to solve for k. The unit of the rate is always mol L⁻¹ t⁻¹, where 't' represents the unit of time (usually seconds, s).
Unit Derivation for Type A Reactions (One Reactant):
- Start with the rate law: Rate = k [A]²
- Solve for k: k = Rate / [A]²
- Substitute units: k = (mol L⁻¹ t⁻¹) / (mol L⁻¹)²
- Simplify: k = L mol⁻¹ t⁻¹
Therefore, for second-order reactions with one reactant, the unit of the rate constant is L mol⁻¹ s⁻¹ (or any other suitable unit of time).
Unit Derivation for Type B Reactions (Two Reactants):
- Start with the rate law: Rate = k [A][B]
- Solve for k: k = Rate / ([A][B])
- Substitute units: k = (mol L⁻¹ t⁻¹) / ((mol L⁻¹)(mol L⁻¹))
- Simplify: k = L² mol⁻² t⁻¹
Hence, for second-order reactions involving two different reactants, the unit of the rate constant becomes L² mol⁻² s⁻¹.
Understanding the Implications of Different Units
The difference in units between Type A and Type B second-order reactions highlights the importance of carefully considering the reaction stoichiometry when determining the rate constant's unit. It's not simply a matter of memorization; it's a direct consequence of how the reaction rate depends on reactant concentrations.
The units themselves provide valuable information. They reflect the dimensions of the rate constant and are essential for verifying the consistency of experimental data with the assumed reaction order. If experimental data yield a rate constant with inconsistent units, it suggests a potential error in the assumed reaction order or in the experimental measurements themselves.
Factors Affecting the Rate Constant (k)
While the units of k are determined by the reaction order, the magnitude of k itself is influenced by several factors:
-
Temperature: The rate constant is highly temperature-dependent. Increasing the temperature usually increases the rate constant exponentially, according to the Arrhenius equation. This relationship is crucial for understanding the temperature sensitivity of chemical reactions.
-
Catalyst: Catalysts accelerate reactions without being consumed. They lower the activation energy, resulting in a larger rate constant at a given temperature.
-
Solvent: The nature of the solvent can significantly impact the rate constant by affecting the stability and reactivity of the reactants and the transition state. The polarity, viscosity, and specific interactions between the solvent and reactants can all play a role.
-
Pressure (for gaseous reactions): The rate constant for gas-phase reactions often depends on pressure. Higher pressure generally increases the concentration of reactant molecules, leading to a faster reaction rate and consequently, a larger rate constant.
Practical Applications and Examples
Understanding the units of rate constants is not merely an academic exercise; it's critical for various practical applications:
-
Reaction Engineering: In chemical engineering, the rate constant is essential for designing and optimizing reactors. Knowing the units helps in correctly scaling up reactions from laboratory to industrial scales.
-
Pharmaceutical Development: The rate constants of drug degradation reactions are crucial in determining shelf life and stability of pharmaceutical products. Precise units ensure accurate prediction of drug degradation over time.
-
Environmental Chemistry: Rate constants are fundamental in environmental modeling, particularly in predicting the fate and transport of pollutants in the environment. Accurate determination of units guarantees reliable predictions.
-
Catalysis Research: In the field of catalysis, the rate constant provides valuable insight into catalytic activity and mechanism. Understanding its units is key to comparing the effectiveness of different catalysts.
Examples of second-order reactions:
-
Saponification: The reaction between an ester and a strong base (like NaOH) to produce a carboxylate salt and an alcohol. This is a classic example of a second-order reaction (Type B).
-
Decomposition of nitrogen dioxide: 2NO₂ → 2NO + O₂. This is another example of a second order reaction (Type A).
-
Many nucleophilic substitution reactions: SN2 reactions in organic chemistry often follow second-order kinetics.
Beyond the Basics: Integrated Rate Laws and Half-Life
The integrated rate laws provide the mathematical relationship between the concentration of reactants and time. For a second-order reaction with one reactant (Type A), the integrated rate law is:
1/[A]<sub>t</sub> = 1/[A]<sub>0</sub> + kt
where:
- [A]<sub>t</sub> is the concentration of A at time t.
- [A]<sub>0</sub> is the initial concentration of A.
The half-life (t<sub>1/2</sub>), the time it takes for the concentration of a reactant to decrease by half, can be determined from the integrated rate law:
t<sub>1/2</sub> = 1 / (k[A]<sub>0</sub>)
Notice how the half-life for a second-order reaction depends on both the rate constant and the initial concentration, unlike first-order reactions. For second-order reactions involving two reactants (Type B), the integrated rate law is more complex and the half-life equation differs.
Conclusion
The unit of the rate constant for a second-order reaction, whether L mol⁻¹ s⁻¹ (for one reactant) or L² mol⁻² s⁻¹ (for two reactants), is not arbitrary; it reflects the inherent dependence of the reaction rate on the concentration(s) of the reactant(s). Understanding these units is paramount in chemical kinetics, facilitating accurate interpretations of experimental data, predictions of reaction behavior, and advancements in related fields. The ability to correctly determine and interpret the units of the rate constant is a fundamental skill for anyone working in chemistry and related disciplines. This understanding is essential for effective problem-solving and a deeper appreciation of reaction dynamics.
Latest Posts
Latest Posts
-
Is The Hydrolysis Of Atp Endergonic Or Exergonic
Apr 02, 2025
-
Choose The Component Parts Of An Amino Acid
Apr 02, 2025
-
Label Each Type Of Intercellular Junction
Apr 02, 2025
-
A Person Throws A Marble Straight Up
Apr 02, 2025
-
Label These Structures Of The Upper Respiratory System
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Unit Of Rate Constant For Second Order Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
