Where On The Periodic Table Are The Nonmetals Located
Muz Play
Mar 31, 2025 · 6 min read
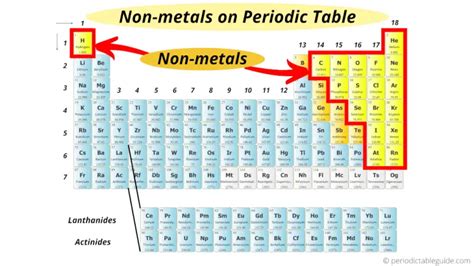
Table of Contents
Where on the Periodic Table are the Nonmetals Located? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its layout is crucial for grasping the behavior of different elements. One key distinction within this organization is the classification of elements into metals, nonmetals, and metalloids. This article delves specifically into the location of nonmetals on the periodic table, explaining their properties and exploring their unique characteristics.
Identifying Nonmetals: A Quick Overview
Before diving into their location, let's establish what defines a nonmetal. Unlike metals, which are typically shiny, conductive, and malleable, nonmetals exhibit contrasting characteristics. They are generally:
- Poor conductors of heat and electricity: This contrasts sharply with the excellent conductivity of metals.
- Brittle: Nonmetals lack the ductility and malleability of metals, shattering instead of bending.
- Low melting and boiling points: Many nonmetals exist as gases at room temperature, while others are low-melting solids.
- Dull appearance: They lack the characteristic luster of metals.
- Gain electrons easily: They tend to form negative ions (anions) in chemical reactions, gaining electrons to achieve a stable electron configuration.
The Geographic Location of Nonmetals on the Periodic Table
Nonmetals are situated on the right-hand side of the periodic table, forming a distinct region. This region is generally separated from the metals by a "stair-step" line running diagonally from boron (B) to astatine (At). Elements bordering this line, exhibiting properties of both metals and nonmetals, are called metalloids or semimetals.
Specifically, the nonmetals are located in:
-
Group 17 (Halogens): This group is characterized by highly reactive elements, including fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They readily form salts with metals. Their reactivity decreases down the group.
-
Group 18 (Noble Gases): These are inert or very unreactive elements due to their full valence electron shells. Helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) are all noble gases. Their lack of reactivity makes them invaluable in various applications.
-
Other Nonmetals: A significant number of nonmetals are scattered throughout the p-block. These include elements like carbon (C), nitrogen (N), oxygen (O), phosphorus (P), sulfur (S), and selenium (Se). These elements are crucial for biological processes and form many important compounds. Hydrogen (H), unique in its properties, is typically placed at the top left of the table, but its nonmetallic behavior places it with this group.
Understanding the Periodic Table's Structure and its Relation to Nonmetal Properties
The periodic table's organization is not arbitrary. It reflects the underlying structure of atoms, particularly the arrangement of electrons in their shells. The properties of elements, including their classification as metals or nonmetals, are directly linked to this electronic configuration.
Electron Configuration and Nonmetal Properties
Nonmetals generally have high electronegativity. This means they have a strong tendency to attract electrons towards themselves in chemical bonds. This high electronegativity stems from their relatively small atomic size and relatively high number of valence electrons (electrons in the outermost shell). To achieve a stable electron configuration (typically an octet, or eight valence electrons), they readily gain electrons, forming negatively charged ions (anions).
Valence Electrons: The Key to Reactivity
The number of valence electrons plays a pivotal role in determining an element's reactivity and its place on the periodic table. Nonmetals, with their near-full valence shells, readily accept electrons to complete their shells, leading to their high reactivity (with the exception of noble gases). This electron-gaining tendency is a defining characteristic of their chemical behavior.
Trends in Nonmetal Properties Across and Down the Periodic Table
As you move across the periodic table (left to right) within a period, the electronegativity of nonmetals generally increases. This reflects the increasing nuclear charge and decreasing atomic size, resulting in a stronger attraction for electrons.
Moving down a group (top to bottom), the electronegativity of nonmetals generally decreases. This is because the increasing distance between the nucleus and the valence electrons weakens the attractive force. The increasing atomic size also plays a role in this trend.
The Importance of Nonmetals in Everyday Life and Industry
Nonmetals, despite their apparent scarcity compared to metals, are essential components of our world, playing crucial roles in various aspects of our lives and industries:
-
Biological Systems: Carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur are fundamental building blocks of life. They form the backbone of carbohydrates, proteins, nucleic acids (DNA and RNA), and lipids, essential for all living organisms.
-
Industrial Applications: Many nonmetals are used extensively in industries:
- Oxygen: Crucial for combustion processes in various industries, including steelmaking and power generation.
- Chlorine: Used extensively in water purification and in the production of various chemicals.
- Nitrogen: A key component of fertilizers, enhancing agricultural productivity.
- Carbon: Essential in materials science, forming the basis of polymers (plastics) and other vital materials.
-
Technological Advancements: Nonmetals are indispensable in numerous technologies:
- Silicon: A crucial element in semiconductors, forming the foundation of modern electronics.
- Fluorine: Used in the production of non-stick coatings and refrigerants.
- Noble gases: Used in lighting, lasers, and other applications, benefiting from their inert nature.
Metalloids: The Bridge Between Metals and Nonmetals
The metalloids, located along the "stair-step" line separating metals and nonmetals, present a fascinating case study. They display properties intermediate between metals and nonmetals, often exhibiting both metallic and nonmetallic characteristics depending on the conditions. Their semiconducting properties make them indispensable in electronics.
Exploring Specific Nonmetal Groups in Detail
Let's delve deeper into the properties and applications of some key nonmetal groups:
Group 17: The Halogens
The halogens are highly reactive nonmetals, existing as diatomic molecules (e.g., Cl₂, Br₂). Their reactivity stems from their high electronegativity and their tendency to gain one electron to achieve a stable octet. Applications include:
- Fluorine: In toothpaste (as fluoride), non-stick cookware, and refrigerants.
- Chlorine: Water purification, bleaching, and chemical synthesis.
- Bromine: In flame retardants and photographic film.
- Iodine: In disinfectants and as an essential element in the human diet (for thyroid hormone production).
Group 18: The Noble Gases
The noble gases, also known as inert gases, exhibit exceptional stability due to their complete valence electron shells. Their lack of reactivity makes them valuable in applications where inertness is crucial:
- Helium: Balloons, cryogenics (cooling to extremely low temperatures).
- Neon: Neon signs, lasers.
- Argon: Welding, preventing oxidation.
- Krypton and Xenon: Lighting applications, lasers.
Other Important Nonmetals
Beyond the halogen and noble gas groups, other vital nonmetals include:
- Carbon: The backbone of organic chemistry, essential in biological molecules, polymers, and graphite/diamond.
- Nitrogen: Fertilizers, explosives, and a component of the Earth's atmosphere.
- Oxygen: Essential for respiration and combustion, supporting life and numerous industrial processes.
- Phosphorus: In fertilizers, matches, and biological molecules (DNA/RNA).
- Sulfur: In vulcanization of rubber, sulfuric acid production, and various other industrial processes.
Conclusion: The Significance of Nonmetal Location and Properties
The location of nonmetals on the periodic table is not arbitrary. Their position reflects their atomic structure and electronic configuration, leading to their characteristic properties. Understanding their location and properties is crucial for grasping their behavior in chemical reactions and their diverse applications in various aspects of science, technology, and everyday life. Their unique characteristics, from the high reactivity of halogens to the inertness of noble gases, highlight the richness and complexity of the periodic table and the elements it encompasses. Further exploration into the properties of individual nonmetals can only deepen our appreciation for their significance in our world.
Latest Posts
Latest Posts
-
Definition Of Social Location In Sociology
Apr 02, 2025
-
Cell Organelles Found In Plant Cell Only
Apr 02, 2025
-
What Is The Difference Between Chemical And Nuclear Reactions
Apr 02, 2025
-
Is The Hydrolysis Of Atp Endergonic Or Exergonic
Apr 02, 2025
-
Choose The Component Parts Of An Amino Acid
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Where On The Periodic Table Are The Nonmetals Located . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
