Does Adding An Inert Gas Affect Equilibrium
Muz Play
Mar 16, 2025 · 6 min read
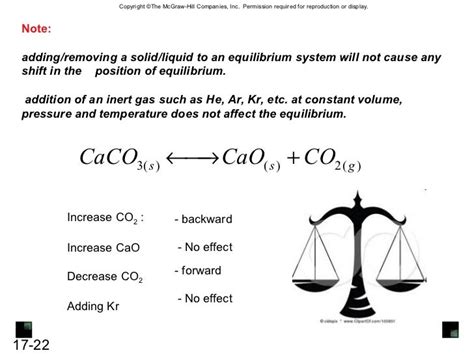
Table of Contents
- Does Adding An Inert Gas Affect Equilibrium
- Table of Contents
- Does Adding an Inert Gas Affect Equilibrium? A Comprehensive Guide
- The Impact of Adding an Inert Gas: A Closer Look
- Constant Volume Conditions
- Constant Pressure Conditions
- Practical Implications and Further Considerations
- Importance of understanding Kp and Kc
- Beyond Ideal Gases: Real-World Scenarios
- The Role of Temperature
- Conclusion: A Recap
- Latest Posts
- Latest Posts
- Related Post
Does Adding an Inert Gas Affect Equilibrium? A Comprehensive Guide
Understanding chemical equilibrium is crucial in chemistry. It's the dynamic state where the rates of the forward and reverse reactions are equal, leading to no net change in the concentrations of reactants and products. A common question that arises is: what happens when an inert gas is added to a system at equilibrium? This seemingly simple addition can have surprising consequences, depending on the conditions of the reaction. This detailed guide will explore the effects of adding an inert gas to a system at equilibrium, considering both constant volume and constant pressure scenarios.
The Impact of Adding an Inert Gas: A Closer Look
The effect of adding an inert gas to a system at equilibrium depends significantly on whether the volume of the system is kept constant or allowed to change. This distinction arises because the partial pressures of the reacting gases are key factors in determining equilibrium position.
Constant Volume Conditions
What happens when we add an inert gas at constant volume? The answer is: nothing. Adding an inert gas at constant volume does not shift the equilibrium position.
Why? Let's consider Le Chatelier's principle, which states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. Adding an inert gas at constant volume increases the total pressure but does not change the partial pressures of the reactants or products. Since the equilibrium constant (K<sub>p</sub> or K<sub>c</sub>) depends only on the partial pressures (or concentrations) of the reactants and products, not on the total pressure, the equilibrium position remains unchanged.
Example: Consider the equilibrium reaction:
N<sub>2</sub>(g) + 3H<sub>2</sub>(g) ⇌ 2NH<sub>3</sub>(g)
If an inert gas is added to this system at constant volume, the total pressure will increase, but the partial pressures of N<sub>2</sub>, H<sub>2</sub>, and NH<sub>3</sub> remain the same. Consequently, the equilibrium position remains undisturbed. The system experiences no stress that requires a shift in equilibrium to alleviate it.
Key takeaway: In a constant volume system, the addition of an inert gas only affects the total pressure, leaving the partial pressures and thus the equilibrium position unaltered.
Constant Pressure Conditions
What happens when we add an inert gas at constant pressure? This scenario is more complex and requires a careful consideration of volume changes.
Understanding the Pressure-Volume Relationship: When an inert gas is added to a system at constant pressure, the total number of moles increases. To maintain constant pressure, the volume of the system must expand. This volume expansion changes the partial pressures of all gaseous components.
The Impact on Equilibrium: The effect on equilibrium depends on the stoichiometry of the reaction.
-
Reactions with a change in the number of moles of gas: If the number of moles of gaseous products differs from the number of moles of gaseous reactants, the equilibrium position will shift. The volume increase caused by the addition of inert gas, effectively lowers the partial pressures of all components. To counteract this decrease in partial pressures, the system will shift to favor the side with a higher number of gas molecules.
-
Reactions without a change in the number of moles of gas: If the number of moles of gaseous reactants equals the number of moles of gaseous products, adding an inert gas at constant pressure will have no effect on the equilibrium position. Although the volume increases and partial pressures decrease, the ratio of partial pressures remains constant. Therefore, the equilibrium position will not shift.
Examples:
- Example 1 (Change in moles of gas): Consider the equilibrium reaction:
N<sub>2</sub>(g) + 3H<sub>2</sub>(g) ⇌ 2NH<sub>3</sub>(g)
This reaction has 4 moles of gas on the reactant side and 2 moles of gas on the product side. Adding an inert gas at constant pressure increases the volume, reducing the partial pressures. The system shifts to the side with more moles of gas, favoring the formation of N<sub>2</sub> and H<sub>2</sub>.
- Example 2 (No change in moles of gas): Consider the equilibrium:
H<sub>2</sub>(g) + I<sub>2</sub>(g) ⇌ 2HI(g)
This reaction has an equal number of moles of gas on both sides. Adding an inert gas at constant pressure will not affect the equilibrium position because the ratio of partial pressures remains unchanged.
Key takeaway: In a constant pressure system, adding an inert gas can shift the equilibrium position only if there is a difference in the number of moles of gaseous reactants and products. The volume expansion resulting from maintaining constant pressure is the crucial factor.
Practical Implications and Further Considerations
The principles discussed above have practical implications in various chemical processes and industries. For instance, understanding the influence of inert gases on equilibrium is essential in optimizing reaction conditions, enhancing yields, and controlling the direction of chemical transformations.
Importance of understanding Kp and Kc
It's crucial to note the distinction between K<sub>p</sub> (equilibrium constant expressed in terms of partial pressures) and K<sub>c</sub> (equilibrium constant expressed in terms of concentrations). While the addition of an inert gas at constant volume does not affect either K<sub>p</sub> or K<sub>c</sub>, changes at constant pressure affect K<sub>p</sub> only because it involves a change in volume. K<sub>c</sub> remains unchanged. This distinction highlights the importance of specifying the conditions (constant volume or constant pressure) when discussing the effects of adding an inert gas.
Beyond Ideal Gases: Real-World Scenarios
The discussion above assumes ideal gas behavior. In reality, gases exhibit non-ideal behavior, particularly at high pressures or low temperatures. In such cases, the effects of adding an inert gas may deviate slightly from the predictions based on ideal gas laws. Intermolecular forces and gas compressibility need to be factored in for more accurate analysis.
The Role of Temperature
Temperature plays a significant role in chemical equilibrium. While the addition of an inert gas at constant volume does not affect temperature, adding it at constant pressure can slightly alter the temperature through expansion work, although this effect is generally negligible. Significant temperature changes, however, can drastically affect the equilibrium position, often overshadowing the effect of added inert gases.
Conclusion: A Recap
The effect of adding an inert gas to a system at equilibrium depends heavily on whether the volume or the pressure is held constant. At constant volume, the addition of an inert gas does not change the equilibrium position because it doesn’t alter the partial pressures of reactants and products. At constant pressure, however, the situation is different. If the number of moles of gaseous products differs from the number of moles of gaseous reactants, the addition of an inert gas will cause a volume expansion, shifting the equilibrium to the side with more gas molecules. If the number of moles is equal on both sides, the addition of an inert gas at constant pressure will not change the equilibrium position. Understanding these principles is critical for controlling chemical reactions and optimizing various industrial processes. The intricacies of real-world systems, incorporating non-ideal gas behavior and temperature effects, further emphasize the need for a detailed and nuanced approach to equilibrium analysis. By thoroughly understanding the impact of inert gases, chemists can refine reaction conditions to achieve desired outcomes and gain deeper insights into chemical dynamics.
Latest Posts
Latest Posts
-
Polalarity Lead To Surface Area Vs
Mar 17, 2025
-
How Do You Find The Domain Of A Composite Function
Mar 17, 2025
-
Proof Of One To One Function
Mar 17, 2025
-
What Is The Plasma Membrane Of A Muscle Fiber Called
Mar 17, 2025
-
How To Find The Equivalence Point Of A Titration
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Adding An Inert Gas Affect Equilibrium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
