Does Ccl4 Have A Dipole Moment
Muz Play
Mar 31, 2025 · 5 min read
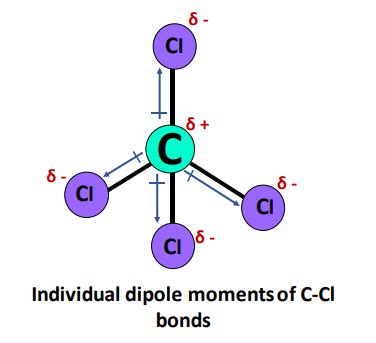
Table of Contents
Does CCl4 Have a Dipole Moment? Understanding Molecular Geometry and Polarity
The question of whether carbon tetrachloride (CCl4) possesses a dipole moment is a fundamental concept in chemistry, bridging the gap between molecular geometry and macroscopic properties. Understanding this requires a grasp of several key ideas: electronegativity, bond polarity, molecular geometry, and the vector nature of dipole moments. This article will delve into these concepts, ultimately answering the question definitively and exploring the broader implications.
Understanding Electronegativity and Bond Polarity
Before tackling the dipole moment of CCl4, let's establish the foundation. Electronegativity is a measure of an atom's ability to attract electrons within a chemical bond. Different atoms exhibit varying electronegativities. When two atoms with different electronegativities bond, the electrons are not shared equally. This unequal sharing creates a polar bond, with a slightly positive end (δ+) and a slightly negative end (δ-). The greater the difference in electronegativity, the more polar the bond.
Chlorine (Cl) is significantly more electronegative than carbon (C). Therefore, each C-Cl bond in CCl4 is polar, with the chlorine atoms carrying a partial negative charge (δ-) and the carbon atom carrying a partial positive charge (δ+). This is crucial in determining the overall dipole moment of the molecule.
Molecular Geometry: The Key to Overall Polarity
While individual bond polarities are important, they don't tell the whole story. The molecular geometry – the three-dimensional arrangement of atoms in a molecule – plays a critical role in determining the overall dipole moment. A dipole moment is a vector quantity, meaning it has both magnitude and direction. Individual bond dipoles act as vectors, and the overall dipole moment is the vector sum of these individual bond dipoles. If these bond dipoles cancel each other out, the molecule will have a net dipole moment of zero.
CCl4 adopts a tetrahedral geometry. The carbon atom is at the center, with four chlorine atoms positioned at the corners of a tetrahedron. This symmetrical arrangement is key to understanding its lack of a dipole moment.
The Cancellation of Bond Dipoles in CCl4
In CCl4, the four C-Cl bonds are arranged symmetrically around the central carbon atom. Each C-Cl bond dipole points from the carbon atom (δ+) towards a chlorine atom (δ-). Because of the tetrahedral geometry, these four bond dipoles are equal in magnitude and point in directions that are symmetrically opposed to each other. Therefore, they perfectly cancel each other out.
Imagine placing four equal-length arrows pointing from the center of a tetrahedron towards each corner. If you carefully add these vectors, you’ll find that their resultant vector sum is zero. This is precisely what happens with the bond dipoles in CCl4.
Therefore, the overall dipole moment of CCl4 is zero. Despite the presence of polar C-Cl bonds, the symmetrical molecular geometry leads to a net cancellation of the bond dipoles.
Comparing CCl4 to Other Molecules
To further illustrate the importance of molecular geometry, let's compare CCl4 to a similar molecule: chloroform (CHCl3). Chloroform also has polar C-Cl bonds, but its molecular geometry is different. It's still tetrahedral, but the replacement of one chlorine atom with a hydrogen atom breaks the symmetry.
In CHCl3, the three C-Cl bond dipoles don't completely cancel out the C-H bond dipole. The net result is a non-zero dipole moment for chloroform. This difference in dipole moment leads to differences in their physical properties, such as boiling points and solubility.
Implications of Zero Dipole Moment in CCl4
The fact that CCl4 has a zero dipole moment has several important implications:
- Nonpolar nature: CCl4 is considered a nonpolar molecule, meaning it doesn't have a significant positive or negative end. This affects its interactions with other molecules.
- Solubility: Nonpolar molecules tend to dissolve in other nonpolar solvents. CCl4, therefore, is soluble in nonpolar solvents but not in polar solvents like water.
- Boiling point: CCl4 has a relatively low boiling point compared to molecules of similar size with dipole moments. The weaker intermolecular forces (London dispersion forces) between nonpolar CCl4 molecules require less energy to overcome during boiling.
- Reactivity: The absence of a significant dipole moment influences the reactivity of CCl4. It's less reactive towards polar reagents compared to molecules with dipole moments.
Advanced Concepts and Further Considerations
The discussion above provides a simplified explanation of dipole moments. In reality, there are more nuanced aspects to consider:
- Bond Lengths: The lengths of the bonds can slightly affect the dipole moment calculation. Slight variations in bond lengths can lead to minor deviations from perfect cancellation.
- Vibrational Effects: Molecules are constantly vibrating. These vibrations can cause temporary changes in bond lengths and angles, which can induce small, transient dipole moments.
- Quantum Mechanical Calculations: Accurate dipole moment calculations require sophisticated quantum mechanical methods. These calculations can provide highly accurate values but are beyond the scope of this introductory discussion.
Conclusion: A Symmetrical Molecule with a Nonpolar Outcome
In conclusion, carbon tetrachloride (CCl4) does not have a dipole moment. This is a direct consequence of its symmetrical tetrahedral geometry, where the four polar C-Cl bond dipoles perfectly cancel each other out. This lack of a dipole moment significantly influences its physical and chemical properties, making it a crucial concept to understand in chemistry. Understanding this concept extends beyond CCl4 to a broader understanding of molecular polarity, a fundamental property influencing intermolecular interactions and reactivity. The careful consideration of electronegativity, bond polarity, and molecular geometry is crucial in predicting the overall polarity of any molecule.
Latest Posts
Latest Posts
-
How To Do Frequency Histogram In Excel
Apr 01, 2025
-
How Are Hydrogen Bonds Different From Covalent
Apr 01, 2025
-
Spicules And Trabeculae Are Found In
Apr 01, 2025
-
Law Of Sines Worksheet And Answers
Apr 01, 2025
-
Northern Blot Southern Blot Western Blot
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Does Ccl4 Have A Dipole Moment . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
