How Are Hydrogen Bonds Different From Covalent
Muz Play
Apr 01, 2025 · 7 min read
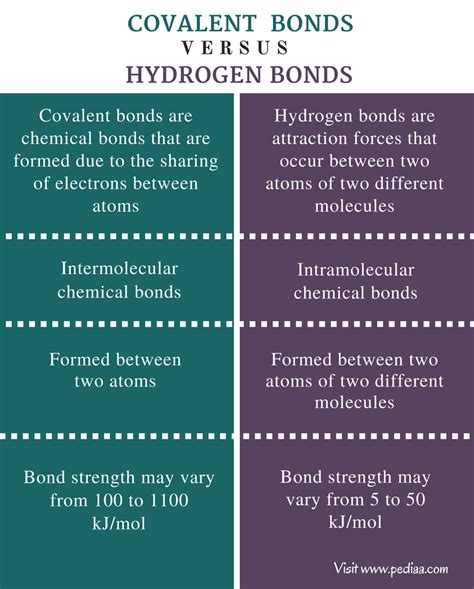
Table of Contents
How Are Hydrogen Bonds Different From Covalent Bonds? A Deep Dive
Understanding the nuances of chemical bonding is crucial for grasping the fundamentals of chemistry and its applications in various fields. While both hydrogen bonds and covalent bonds contribute significantly to the structure and properties of molecules, they differ significantly in their nature, strength, and implications. This article delves into the core differences between these two types of bonds, exploring their formation, characteristics, and roles in biological systems and materials science.
What are Covalent Bonds?
Covalent bonds represent a fundamental type of chemical bond where atoms share one or more pairs of electrons to achieve a stable electron configuration, typically resembling a noble gas. This sharing occurs between atoms with similar electronegativities, meaning they have a relatively equal pull on the shared electrons. The strength of a covalent bond is determined by the number of shared electron pairs and the distance between the nuclei of the bonded atoms. A single covalent bond involves one shared electron pair, a double bond involves two pairs, and a triple bond involves three pairs. The strength of these bonds increases with the number of shared pairs.
Characteristics of Covalent Bonds:
- Strong Bonds: Covalent bonds are relatively strong bonds, requiring significant energy to break. This strength contributes to the stability of molecules held together by these bonds.
- Directional Bonds: The shared electron pairs are localized between the participating atoms, resulting in a directional bond. This directionality plays a crucial role in determining the molecular geometry and overall shape of molecules.
- Non-Conductivity: Covalent compounds, generally, do not conduct electricity in either solid or liquid states because they lack free-moving charged particles (ions). Exceptions exist in the case of certain molecules that can ionize in solution.
- Low Melting and Boiling Points: Covalent compounds typically have lower melting and boiling points compared to ionic compounds due to the weaker intermolecular forces between the molecules. However, network covalent structures (like diamond) are exceptions, possessing exceptionally high melting points.
- Solubility: The solubility of covalent compounds varies considerably depending on the polarity of the molecule and the polarity of the solvent. Polar covalent molecules tend to dissolve readily in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents.
Examples of Covalent Bonds:
- Water (H₂O): Oxygen shares electrons with two hydrogen atoms, forming two single covalent bonds.
- Methane (CH₄): Carbon shares electrons with four hydrogen atoms, forming four single covalent bonds.
- Ethylene (C₂H₄): Two carbon atoms are double-bonded to each other, while each carbon atom forms two additional single bonds with hydrogen atoms.
- Diamond: A giant covalent structure where each carbon atom is covalently bonded to four other carbon atoms in a tetrahedral arrangement.
What are Hydrogen Bonds?
Hydrogen bonds are a special type of intermolecular force, significantly weaker than covalent bonds. They occur when a hydrogen atom covalently bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a different molecule or even within the same molecule. This attraction is due to the large difference in electronegativity between the hydrogen atom and the electronegative atom it's bonded to. The hydrogen atom, being partially positively charged (δ+), is attracted to the partially negatively charged (δ−) lone pairs of electrons on the other electronegative atom.
Characteristics of Hydrogen Bonds:
- Weak Bonds: Hydrogen bonds are significantly weaker than covalent bonds. They are easily broken and reformed under normal conditions.
- Non-directional (relatively): While not completely non-directional, hydrogen bonds are less directional than covalent bonds. The strength of a hydrogen bond can vary slightly depending on the orientation of the molecules involved.
- Impact on Properties: Despite their relative weakness, hydrogen bonds have a profound influence on the physical and chemical properties of many substances, particularly water.
- Important in Biological Systems: Hydrogen bonds play critical roles in various biological processes, including protein folding, DNA structure, and enzyme-substrate interactions.
Examples of Hydrogen Bonds:
- Water (H₂O): Hydrogen bonds form between the partially positive hydrogen atoms of one water molecule and the partially negative oxygen atoms of another water molecule. This extensive hydrogen bonding network gives water its unique properties, including high boiling point, surface tension, and excellent solvent capabilities.
- DNA: Hydrogen bonds link the complementary base pairs (adenine-thymine and guanine-cytosine) in the DNA double helix. These bonds allow for the easy separation and replication of the DNA strands during cell division.
- Proteins: Hydrogen bonds stabilize the secondary structures of proteins (alpha-helices and beta-sheets) and contribute to their overall three-dimensional structure.
Key Differences Between Covalent and Hydrogen Bonds:
The following table summarizes the key distinctions between covalent and hydrogen bonds:
| Feature | Covalent Bond | Hydrogen Bond |
|---|---|---|
| Nature | Intramolecular (within a molecule) | Intermolecular (between molecules) |
| Strength | Strong | Weak |
| Electron Sharing | Electrons are shared between atoms | No electron sharing; electrostatic attraction |
| Formation | Sharing of valence electrons | Attraction between a partially positive hydrogen and a partially negative atom |
| Bond Energy | High (hundreds of kJ/mol) | Low (tens of kJ/mol) |
| Directionality | Highly directional | Relatively less directional |
| Impact on Properties | Influences molecular shape and stability | Influences boiling point, melting point, solubility, and other physical properties |
| Examples | Water (O-H bonds), methane (C-H bonds) | Water (between water molecules), DNA (base pairing) |
The Significance of Hydrogen Bonds in Various Contexts
Hydrogen bonds, despite being weaker than covalent bonds, exert a considerable influence on the macroscopic properties of substances and play vital roles in biological systems.
Water's Unique Properties:
The exceptional properties of water—high boiling point, high surface tension, high specific heat capacity, and its role as an excellent solvent—are all directly attributable to the extensive network of hydrogen bonds between water molecules. These properties are crucial for sustaining life on Earth.
Biological Macromolecules:
Hydrogen bonds are essential for maintaining the three-dimensional structures of biological macromolecules like proteins and nucleic acids (DNA and RNA). In proteins, hydrogen bonds stabilize secondary structures such as alpha-helices and beta-sheets. These secondary structures then fold into complex tertiary structures, which are essential for protein function. Similarly, in DNA, hydrogen bonds between base pairs hold the two strands of the double helix together, allowing for DNA replication and transcription.
Material Science:
Hydrogen bonding also plays a significant role in material science. The properties of many materials, including polymers and gels, are heavily influenced by hydrogen bonding interactions. Understanding and manipulating these interactions allows for the design of materials with specific properties, such as strength, elasticity, and biocompatibility.
Other Applications:
Hydrogen bonding is relevant in numerous other fields, including:
- Drug design: Understanding hydrogen bonding is crucial for designing drugs that can effectively bind to their target molecules.
- Crystal engineering: The strength and directionality of hydrogen bonds play an important role in the formation of crystalline structures.
- Surface science: Hydrogen bonding influences the interactions of molecules with surfaces.
Conclusion: A Tale of Two Bonds
In conclusion, while both covalent and hydrogen bonds are crucial in chemistry, their differences are significant. Covalent bonds represent a strong intramolecular interaction involving the sharing of electron pairs, resulting in stable molecules. Hydrogen bonds, on the other hand, are relatively weaker intermolecular interactions that arise from electrostatic attraction between a partially positive hydrogen atom and a partially negative atom. While weaker, hydrogen bonds exert a disproportionately large influence on the physical properties of substances and play critical roles in various biological and material science applications. Understanding the distinct characteristics of both types of bonds is essential for comprehending the diverse properties of matter and the complexities of life itself. The ability to discern between these bond types allows scientists to predict molecular behavior and develop new materials with tailored characteristics. Further research into both covalent and hydrogen bonds continues to unveil their multifaceted nature and broaden their applications in numerous scientific fields.
Latest Posts
Latest Posts
-
Difference Between Light Independent And Light Dependent Reactions
Apr 02, 2025
-
How Many Neutrons Does Be Have
Apr 02, 2025
-
Compared To The Nervous System The Endocrine System
Apr 02, 2025
-
The Structural And Functional Unit Of The Kidney Is The
Apr 02, 2025
-
What Is The Moment Of Inertia Of Particle A
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Are Hydrogen Bonds Different From Covalent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
