Does Electronegativity Decrease Down A Group
Muz Play
Mar 23, 2025 · 5 min read
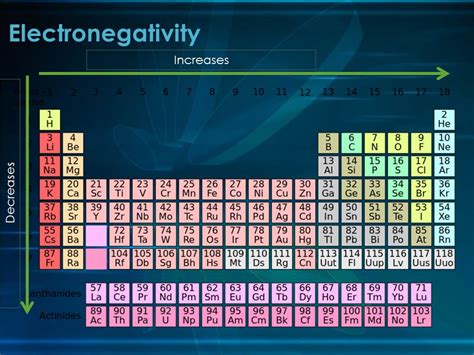
Table of Contents
Does Electronegativity Decrease Down a Group? A Deep Dive into Periodic Trends
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract electrons within a chemical bond. Understanding how electronegativity changes across the periodic table is crucial for predicting the properties of molecules and compounds. A common question that arises is: does electronegativity decrease down a group? The short answer is yes, but understanding why this trend exists requires a deeper exploration of atomic structure and bonding.
Understanding Electronegativity
Before delving into the periodic trend, let's solidify our understanding of electronegativity itself. It's a measure of the relative attraction an atom has for shared electrons in a covalent bond. Atoms with high electronegativity strongly pull electrons towards themselves, creating polar bonds. Conversely, atoms with low electronegativity have a weaker pull on shared electrons.
Several scales exist to quantify electronegativity, the most common being the Pauling scale. While the absolute values vary slightly between scales, the relative differences between elements remain consistent, allowing for accurate comparisons and predictions.
Several factors influence an atom's electronegativity:
- Nuclear Charge: A higher positive charge in the nucleus attracts electrons more strongly.
- Atomic Radius: A larger atomic radius means the valence electrons are further from the nucleus, experiencing a weaker attractive force.
- Shielding Effect: Inner electrons shield the valence electrons from the full positive charge of the nucleus, reducing the effective nuclear charge experienced by the valence electrons.
The Downward Trend: Why Electronegativity Decreases Down a Group
The periodic trend of electronegativity decreasing down a group is a direct consequence of the interplay between atomic radius and shielding effect. As we move down a group, the following occurs:
1. Increasing Atomic Radius: The Dominant Factor
As we descend a group, each successive element adds another electron shell. This significantly increases the atomic radius. The valence electrons are now further away from the positively charged nucleus. This increased distance weakens the electrostatic attraction between the nucleus and the valence electrons, making them less tightly held and consequently reducing the atom's ability to attract shared electrons in a bond. This is the primary reason for the decrease in electronegativity down a group.
2. Increasing Shielding Effect: An Amplifying Factor
Along with the increasing atomic radius, the number of inner electrons (core electrons) also increases down a group. These inner electrons shield the valence electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons, further diminishing the attraction between the nucleus and the valence electrons. Therefore, the shielding effect acts as an amplifying factor to the decrease in electronegativity.
3. The Combined Effect: A Clear Trend
The combined effect of the increasing atomic radius and the increasing shielding effect leads to a clear and consistent decrease in electronegativity as we move down a group in the periodic table. The influence of the increased atomic radius is generally more significant than the increase in the number of protons.
Exceptions and Nuances
While the general trend of decreasing electronegativity down a group holds true, minor exceptions or deviations might be observed in certain cases, particularly when comparing elements within the same period (row). These exceptions often arise from complex electronic configurations or other subtle factors influencing atomic behavior. However, the overall trend remains remarkably consistent.
For instance, the electronegativity values might not show a perfectly linear decrease. Small irregularities can occur due to variations in electron-electron repulsions or other factors affecting electron distribution within the atom.
Furthermore, the electronegativity of certain elements, especially those with partially filled d or f orbitals, can be influenced by factors beyond the simple atomic radius and shielding effects. These factors can lead to some slight irregularities in the overall trend.
Illustrative Examples: Comparing Groups
Let's consider some examples to illustrate this trend:
Group 1 (Alkali Metals):
- Lithium (Li): Electronegativity ≈ 0.98
- Sodium (Na): Electronegativity ≈ 0.93
- Potassium (K): Electronegativity ≈ 0.82
- Rubidium (Rb): Electronegativity ≈ 0.82
- Caesium (Cs): Electronegativity ≈ 0.79
As we move down Group 1, the electronegativity consistently decreases. This reflects the increasing atomic radius and shielding effect, leading to weaker attraction of valence electrons.
Group 17 (Halogens):
- Fluorine (F): Electronegativity ≈ 3.98
- Chlorine (Cl): Electronegativity ≈ 3.16
- Bromine (Br): Electronegativity ≈ 2.96
- Iodine (I): Electronegativity ≈ 2.66
- Astatine (At): Electronegativity ≈ 2.2
Again, a clear downward trend in electronegativity is observed, highlighting the dominance of atomic radius and shielding effects.
Implications of Decreasing Electronegativity Down a Group
The decrease in electronegativity down a group has significant implications for chemical bonding and reactivity:
- Bond Polarity: Elements at the bottom of a group form less polar bonds compared to elements at the top.
- Reactivity: Elements with lower electronegativity are generally more reactive, especially in reactions involving electron donation.
- Compound Properties: The electronegativity difference between elements in a compound dictates the type and strength of bonds formed, influencing the compound's physical and chemical properties (e.g., melting point, boiling point, solubility).
Conclusion: A Fundamental Periodic Trend
In conclusion, the decrease in electronegativity down a group is a fundamental and predictable periodic trend driven primarily by the increase in atomic radius and the amplified effect of increased shielding. While minor deviations might occur, the overall trend remains robust and crucial for understanding the behavior of elements and their compounds. Understanding this trend provides valuable insight into the properties of matter and the nature of chemical bonding. This knowledge is indispensable for chemists, materials scientists, and anyone working with chemical systems. The consistent decrease in electronegativity down a group serves as a testament to the powerful organizing principle embodied by the periodic table itself.
Latest Posts
Latest Posts
-
Conflict Theorists View Gender Differences As
Mar 24, 2025
-
What Is The Subtraction Property Of Equality
Mar 24, 2025
-
Relation Between Electric Field And Potential
Mar 24, 2025
-
Campinha Bacote Model Of Cultural Competence
Mar 24, 2025
-
Formula For Mann Whitney U Test
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Does Electronegativity Decrease Down A Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
