Does Electronegativity Increase Down A Group
Muz Play
Mar 21, 2025 · 6 min read
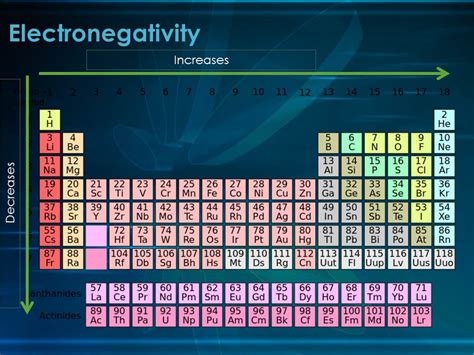
Table of Contents
Does Electronegativity Increase Down a Group? A Deep Dive into Periodic Trends
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract electrons towards itself within a chemical bond. Understanding how electronegativity changes across the periodic table is crucial for predicting molecular properties, reactivity, and bond polarity. A common question that arises is: does electronegativity increase down a group? The short answer is no, it generally decreases. This article will delve into the reasons behind this trend, exploring the underlying principles and providing illustrative examples.
Understanding Electronegativity
Before diving into the group trend, let's solidify our understanding of electronegativity itself. Electronegativity isn't a directly measurable quantity like mass or charge. Instead, it's a relative property, representing the tendency of an atom to attract bonding electrons. Several scales exist to quantify electronegativity, the most common being the Pauling scale. On this scale, fluorine (F), the most electronegative element, is assigned a value of 4.0. Other elements are then assigned values relative to fluorine.
Factors Affecting Electronegativity
Several factors contribute to an atom's electronegativity:
-
Nuclear Charge: A higher nuclear charge (more protons) generally increases the attraction for electrons. The stronger the positive pull from the nucleus, the more strongly it can attract electrons involved in bonding.
-
Atomic Radius: As atomic radius increases, the distance between the nucleus and the valence electrons grows. This increased distance weakens the attractive force of the nucleus on the bonding electrons, reducing electronegativity.
-
Shielding Effect: Inner electrons shield the valence electrons from the full positive charge of the nucleus. The more inner electrons (and electron shells), the greater the shielding effect, reducing the effective nuclear charge experienced by the valence electrons and consequently lowering electronegativity.
The Trend Down a Group: Decreasing Electronegativity
Now, let's address the core question: why does electronegativity decrease down a group?
As you move down a group in the periodic table, the atomic number increases. This means there are more protons in the nucleus, which, in isolation, would suggest increased electronegativity. However, two other factors counteract this increase in nuclear charge, leading to a net decrease in electronegativity:
1. Increased Atomic Radius: The dominant factor influencing the downward trend is the significant increase in atomic radius. As you move down a group, new electron shells are added. These additional shells significantly increase the distance between the nucleus and the valence electrons. This greater distance diminishes the electrostatic attraction between the nucleus and the valence electrons, thus reducing the atom's ability to attract electrons in a bond – lowering electronegativity.
2. Increased Shielding Effect: The addition of electron shells also increases the shielding effect. The inner electrons effectively screen the valence electrons from the positive charge of the nucleus. This shielding reduces the effective nuclear charge experienced by the valence electrons, further weakening the attraction for bonding electrons and contributing to the decrease in electronegativity.
Illustrative Example: Group 17 (Halogens)
Let's consider Group 17, the halogens, to illustrate this trend:
-
Fluorine (F): Has a small atomic radius and a relatively weak shielding effect. This results in a high electronegativity (4.0).
-
Chlorine (Cl): Has a larger atomic radius and a stronger shielding effect than fluorine, leading to lower electronegativity (3.0).
-
Bromine (Br): Even larger atomic radius and stronger shielding than chlorine, resulting in even lower electronegativity (2.8).
-
Iodine (I): The largest atomic radius and strongest shielding effect in this group, exhibiting the lowest electronegativity (2.5).
This progressive decrease in electronegativity down Group 17 is a clear demonstration of the overriding influence of increased atomic radius and shielding effect over the increase in nuclear charge.
Exceptions and Nuances
While the general trend of decreasing electronegativity down a group is well-established, there can be subtle exceptions and nuances. These deviations are often due to specific electronic configurations or relativistic effects in heavier elements. For instance, the electronegativity values might not show a perfectly linear decrease. Small irregularities can arise from variations in electron-electron repulsions or other subtle electronic interactions within the atom. However, the overall downward trend remains consistent.
Consequences of Decreasing Electronegativity Down a Group
The decrease in electronegativity down a group has significant implications for the chemical properties and reactivity of elements:
-
Bond Polarity: Elements with lower electronegativity form less polar bonds with other elements.
-
Reactivity: The reactivity of elements within a group often correlates with electronegativity. Elements with lower electronegativity are generally less reactive than those with higher electronegativity.
-
Oxidation States: The preferred oxidation states of elements can be influenced by their electronegativity. Elements with lower electronegativity tend to exhibit lower oxidation states.
-
Acid-Base Properties: Electronegativity plays a crucial role in determining the acidic or basic nature of compounds.
Comparing Electronegativity Trends Across Periods and Groups
It's important to contrast the electronegativity trend down a group with the trend across a period (left to right). Across a period, electronegativity generally increases. This is primarily due to the increasing nuclear charge with only a gradual increase in atomic radius and shielding effect. The combination of a stronger nuclear pull and relatively minor increases in size and shielding results in a net increase in electronegativity across a period.
Applications and Importance of Understanding Electronegativity Trends
Understanding electronegativity trends is paramount in various areas of chemistry and related fields:
-
Predicting Bond Polarity: Knowing the electronegativity difference between two atoms allows us to predict the polarity of the bond they form. This is essential for understanding molecular properties, such as dipole moments and intermolecular forces.
-
Understanding Chemical Reactivity: Electronegativity helps predict the reactivity of elements and compounds. Elements with high electronegativity tend to be strong oxidizing agents, while those with low electronegativity are often strong reducing agents.
-
Designing and Synthesizing New Materials: Understanding electronegativity is crucial in designing new materials with specific properties. For example, tailoring the electronegativity of components can lead to materials with enhanced conductivity, catalytic activity, or other desired features.
-
Interpreting Spectroscopic Data: Electronegativity influences the electronic structure and bonding of molecules, which is reflected in spectroscopic data (IR, NMR, UV-Vis). Knowing electronegativity trends aids in interpreting and assigning spectral peaks.
Conclusion: Electronegativity and the Periodic Table
In summary, electronegativity generally decreases down a group in the periodic table. This decrease is primarily attributed to the overwhelming influence of increased atomic radius and shielding effect, which counteract the increasing nuclear charge. Understanding this trend, along with the contrasting trend across periods, is fundamental to comprehending the chemical behavior of elements and predicting the properties of compounds. The impact of electronegativity extends to various applications across chemistry and related fields, making it a cornerstone concept in chemical understanding. While minor exceptions exist, the overall downward trend remains a robust and reliable periodic property.
Latest Posts
Latest Posts
-
The Defining Trait Of Hominins Is
Mar 28, 2025
-
What Is The Electron Configuration Of Li
Mar 28, 2025
-
Is Rusting Iron A Physical Or Chemical Change
Mar 28, 2025
-
Do Both Eukaryotes And Prokaryotes Have Ribosomes
Mar 28, 2025
-
Difference Between Gage Pressure And Absolute Pressure
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Does Electronegativity Increase Down A Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
