What Is The Electron Configuration Of Li
Muz Play
Mar 28, 2025 · 6 min read
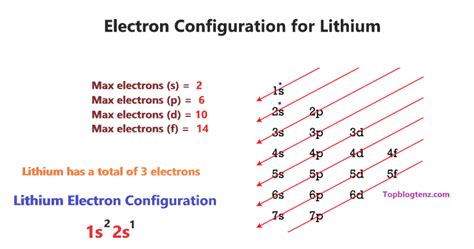
Table of Contents
What is the Electron Configuration of Li? A Deep Dive into Lithium's Atomic Structure
Lithium (Li), the lightest alkali metal, holds a significant place in chemistry and various technological applications. Understanding its electron configuration is fundamental to grasping its chemical behavior and properties. This article will provide a comprehensive exploration of lithium's electron configuration, explaining the underlying principles and delving into its implications.
Understanding Electron Configuration
Before diving into lithium's specific configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons within an atom's electron shells and subshells. It dictates how an atom will interact with other atoms, forming chemical bonds and determining its overall chemical reactivity. This arrangement is governed by the principles of quantum mechanics, specifically the Pauli Exclusion Principle and Hund's Rule.
The Pauli Exclusion Principle
This fundamental principle states that no two electrons in an atom can have the same set of four quantum numbers. These quantum numbers describe an electron's state:
-
Principal Quantum Number (n): Represents the electron shell's energy level (n = 1, 2, 3...). Higher 'n' values indicate higher energy levels and greater distance from the nucleus.
-
Azimuthal Quantum Number (l): Describes the subshell's shape (l = 0 to n-1). l = 0 corresponds to an s subshell (spherical), l = 1 to a p subshell (dumbbell-shaped), l = 2 to a d subshell, and so on.
-
Magnetic Quantum Number (ml): Specifies the orbital's orientation within a subshell (ml = -l to +l). For example, a p subshell (l = 1) has three orbitals (ml = -1, 0, +1).
-
Spin Quantum Number (ms): Indicates the electron's spin, either +1/2 (spin up) or -1/2 (spin down).
Hund's Rule
Hund's Rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
Determining Lithium's Electron Configuration
Lithium has an atomic number of 3, meaning it possesses 3 protons and 3 electrons in its neutral state. To determine its electron configuration, we systematically fill the orbitals according to the principles mentioned above, starting with the lowest energy levels:
-
The first shell (n=1): This shell contains only one subshell, the 1s subshell, which can hold a maximum of two electrons. Therefore, two of lithium's electrons fill the 1s orbital.
-
The second shell (n=2): This shell contains the 2s and 2p subshells. The 2s subshell can accommodate two electrons, while the 2p subshell can hold up to six electrons. The remaining electron from lithium fills the 2s orbital.
Therefore, the complete electron configuration of lithium is 1s²2s¹. This concise notation signifies that two electrons occupy the 1s orbital and one electron occupies the 2s orbital.
Visualizing Lithium's Electron Configuration
A visual representation can aid understanding. We can depict lithium's electron configuration using orbital diagrams:
1s: ↑↓
2s: ↑
2p: _ _ _ (empty)
Each arrow represents an electron, with opposite arrows indicating opposite spins. The empty 2p orbitals highlight that lithium's valence electron resides in the 2s orbital.
Implications of Lithium's Electron Configuration
Lithium's electron configuration directly influences its chemical properties and reactivity.
Chemical Reactivity
The single electron in the 2s orbital is relatively loosely held, making it readily available for chemical bonding. This explains lithium's high reactivity, particularly with nonmetals like halogens (e.g., chlorine, fluorine). Lithium readily loses this valence electron to achieve a stable noble gas configuration, similar to helium (1s²), forming a +1 ion (Li⁺).
Ionization Energy
The ionization energy is the energy required to remove an electron from an atom. Lithium's relatively low first ionization energy reflects the ease with which it loses its outer electron. Subsequent ionization energies are significantly higher, as they involve removing electrons from the more tightly bound inner shell.
Bonding Characteristics
Because of its single valence electron, lithium forms predominantly ionic bonds, donating its electron to electronegative atoms to form stable ionic compounds like lithium chloride (LiCl). While it can participate in covalent bonding, it's less common than ionic bonding for lithium.
Lithium in Various Applications
Understanding lithium's unique electron configuration provides insight into its widespread applications in various fields:
Batteries
Lithium's high reactivity and low ionization energy make it ideal for rechargeable batteries. The ease with which lithium ions (Li⁺) can move between electrodes underpins the functionality of lithium-ion batteries, which are ubiquitous in portable electronics and electric vehicles. The demand for lithium-ion batteries is driving significant research into efficient lithium extraction and battery technology optimization.
Medicine
Lithium compounds have been used in the treatment of certain mental illnesses, particularly bipolar disorder. The exact mechanism of action is not fully understood, but it's believed to involve interactions with neurotransmitters in the brain.
Ceramics and Glass
Lithium oxide (Li₂O) is added to ceramics and glass to enhance their properties. It lowers the melting point, increases strength, and improves chemical resistance. This makes lithium-containing ceramics and glass suitable for various high-performance applications.
Lubricants
Lithium-based greases are excellent lubricants due to their high-temperature stability and resistance to oxidation. Their unique properties make them suitable for diverse applications in industries ranging from automotive to aerospace.
Advanced Concepts and Further Exploration
While the basic electron configuration provides a fundamental understanding, a deeper investigation can delve into more nuanced aspects:
Excited States
Under specific conditions, like exposure to electromagnetic radiation, lithium's electron can be promoted to a higher energy level, resulting in an excited state. These excited states have different electron configurations and contribute to the emission spectra observed in lithium.
Isotopes
Lithium exists in two stable isotopes, ⁷Li and ⁶Li, differing in neutron count. While the electron configuration remains the same for both isotopes, their nuclear properties vary, impacting their use in specific applications (e.g., nuclear fusion).
Quantum Mechanical Calculations
Advanced quantum mechanical calculations can provide a more precise description of lithium's electron distribution, including the effects of electron-electron correlation and relativistic effects. These refined calculations offer a more accurate depiction of the atom's properties.
Conclusion
Lithium's simple yet crucial electron configuration (1s²2s¹) directly dictates its fundamental chemical and physical properties. This configuration explains lithium's reactivity, its preference for forming ionic bonds, and its low ionization energy. Understanding this simple structure allows us to grasp the widespread applications of lithium in diverse technologies and fields, emphasizing the significance of its atomic structure in shaping its role in the modern world. Further exploration of advanced concepts allows for a richer understanding of lithium's behavior and potential.
Latest Posts
Latest Posts
-
Is Water An Ionic Or Covalent Bond
Mar 31, 2025
-
Why Would A Beta Sheet Not Have Alternating Polar Nonpolar Aa
Mar 31, 2025
-
What Does A Chemical Equation Describe
Mar 31, 2025
-
How Do You Divide A Whole Number
Mar 31, 2025
-
Is Breaking A Bond Endothermic Or Exothermic
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Li . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
