Is Water An Ionic Or Covalent Bond
Muz Play
Mar 31, 2025 · 5 min read
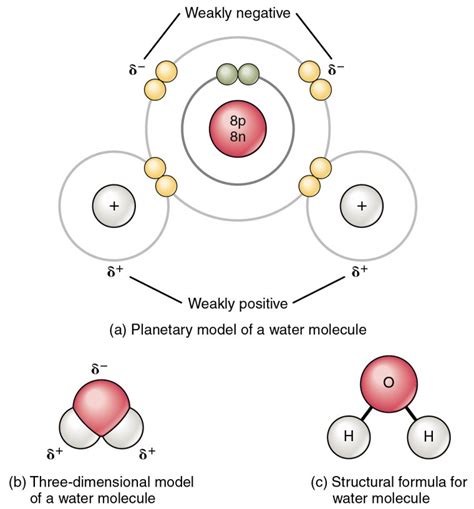
Table of Contents
Is Water an Ionic or Covalent Bond? Understanding the Nature of Water's Bonds
Water, the elixir of life, is a seemingly simple molecule composed of two hydrogen atoms and one oxygen atom (H₂O). However, the nature of the bonds holding these atoms together is a fundamental concept in chemistry, often sparking discussions and confusion. Is water held together by ionic bonds or covalent bonds? The answer, while seemingly straightforward, unveils a fascinating depth concerning the properties and behavior of this crucial substance. This article delves deep into the intricacies of water's bonding, exploring the differences between ionic and covalent bonds, examining the specific type of covalent bond found in water, and exploring the consequences of this bonding on water's unique properties.
The Fundamentals: Ionic vs. Covalent Bonds
Before diving into the specifics of water, let's establish a clear understanding of the two main types of chemical bonds: ionic and covalent.
Ionic Bonds: The Transfer of Electrons
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This occurs when one atom, typically a metal, readily loses one or more electrons (becoming a positively charged cation), and another atom, usually a non-metal, readily gains these electrons (becoming a negatively charged anion). The strong electrostatic force of attraction between these oppositely charged ions creates the ionic bond. Think of it as a transfer of ownership – one atom gives up electrons, and another accepts them. Classic examples include sodium chloride (NaCl, table salt) and magnesium oxide (MgO). These bonds are generally strong and result in crystalline structures with high melting and boiling points.
Covalent Bonds: The Sharing of Electrons
In contrast to ionic bonds, covalent bonds involve the sharing of electrons between atoms. This sharing occurs when atoms achieve a more stable electron configuration by sharing electrons in their outermost shell (valence shell). Covalent bonds typically form between non-metal atoms, where the electronegativity difference between the atoms is relatively small. This means neither atom is significantly more likely to completely steal electrons from the other. Instead, they compromise and share to achieve stability. The shared electrons are attracted to the nuclei of both atoms, holding them together. Examples include methane (CH₄) and carbon dioxide (CO₂). The strength of covalent bonds can vary, impacting the substance's physical properties.
The Covalent Bond in Water: A Deeper Dive
Now, let's focus on the specific type of bond that holds water molecules together. Water is formed by a covalent bond between one oxygen atom and two hydrogen atoms. However, it's not just any covalent bond; it's a polar covalent bond.
Polar Covalent Bonds: Unequal Sharing
A polar covalent bond arises when the atoms involved in the bond have different electronegativities. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Oxygen is significantly more electronegative than hydrogen. This means oxygen has a stronger pull on the shared electrons in the O-H bonds. As a result, the shared electrons spend more time closer to the oxygen atom, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This unequal distribution of charge creates a dipole moment, making the water molecule polar.
The Significance of Polarity
The polarity of water is crucial to understanding its unique properties. Because of its polarity, water molecules are strongly attracted to each other through hydrogen bonds. Hydrogen bonds are a special type of intermolecular force (a force between molecules, not within a molecule like covalent bonds) where a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen) is attracted to another electronegative atom in a nearby molecule. These hydrogen bonds are relatively weak compared to covalent bonds, but collectively they are responsible for many of water's remarkable characteristics.
The Unique Properties of Water: A Consequence of its Bonding
Water's polar covalent bonding and subsequent hydrogen bonding lead to a suite of unique properties essential for life:
-
High Boiling Point: The hydrogen bonds between water molecules require significant energy to overcome, resulting in a surprisingly high boiling point (100°C) compared to other molecules of similar size.
-
High Specific Heat Capacity: Water can absorb a large amount of heat with a relatively small temperature change. This property is crucial for regulating temperature in organisms and in the environment.
-
High Surface Tension: The strong hydrogen bonds create a cohesive force at the surface of water, resulting in high surface tension. This allows certain insects to walk on water.
-
Excellent Solvent: Water's polarity allows it to dissolve many ionic and polar substances, making it an excellent solvent for various biological and chemical processes. "Like dissolves like" – polar substances dissolve polar substances.
-
Density Anomaly: Ice is less dense than liquid water, a unique property that protects aquatic life during freezing temperatures. This is due to the specific arrangement of water molecules in ice, forming a more open crystalline structure.
-
Universal Solvent: Due to its polarity and ability to form hydrogen bonds, water's solvating power is remarkably high, earning it the title of "universal solvent." This allows it to dissolve and transport various substances crucial for biological processes.
Debunking Misconceptions: Why Water Isn't Ionic
While some might mistakenly consider water an ionic compound because of its polar nature and the resulting charge separation, it's crucial to remember that there's no complete transfer of electrons between oxygen and hydrogen. The electrons are shared, albeit unequally, forming a covalent bond. The partial charges are not full ionic charges; they are a consequence of the unequal sharing, not a complete transfer of electrons. The presence of partial charges doesn't transform the fundamental nature of the bond from covalent to ionic.
Conclusion: The Power of Polar Covalent Bonds in Water
In conclusion, water is unequivocally held together by polar covalent bonds, not ionic bonds. The unequal sharing of electrons between oxygen and hydrogen atoms creates a polar molecule with unique properties arising from its ability to form strong hydrogen bonds. These properties are not only fundamental to the existence of life as we know it but also crucial for numerous applications in various fields of science and technology. Understanding the nature of water's bonding is pivotal to appreciating the multifaceted role it plays in our world. The seemingly simple H₂O molecule reveals a complex and fascinating interplay of atomic forces, highlighting the elegance and importance of chemical bonding.
Latest Posts
Latest Posts
-
When Does The Segregation Of Alleles Occur
Apr 01, 2025
-
Cardiac Muscles Differ From Skeletal Muscles In That They
Apr 01, 2025
-
Closing Entries Are Journalized And Posted
Apr 01, 2025
-
How To Calculate Parts Per Thousand
Apr 01, 2025
-
Which Of The Following Statements Pertaining To Asthma Is False
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Water An Ionic Or Covalent Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
