Does Gas Have A Fixed Volume
Muz Play
Mar 24, 2025 · 7 min read
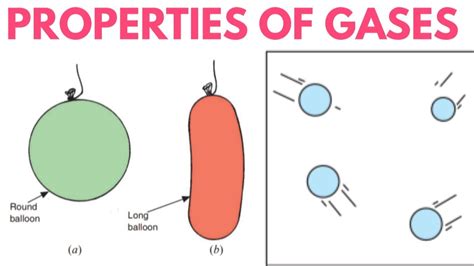
Table of Contents
Does Gas Have a Fixed Volume? Exploring the Properties of Gases
The question of whether gas has a fixed volume is a fundamental one in understanding the behavior of matter. The answer, simply put, is no. Unlike solids and liquids, gases do not possess a fixed volume. Their volume is highly dependent on external factors such as pressure and temperature. This characteristic is a key differentiator and forms the basis of many scientific principles and applications. This article delves deep into the properties of gases, explaining why they lack a fixed volume and exploring the related concepts that govern their behavior.
Understanding the States of Matter: Solid, Liquid, and Gas
Before diving into the specifics of gas volume, it's crucial to understand the three fundamental states of matter: solid, liquid, and gas. Each state exhibits distinct properties based on the arrangement and interaction of its constituent particles (atoms or molecules).
Solids: Fixed Volume and Shape
Solids possess both a fixed volume and a fixed shape. The particles in a solid are tightly packed together in a highly ordered arrangement, held in place by strong intermolecular forces. This strong attraction restricts the movement of particles, resulting in the rigid structure and unchanging volume characteristic of solids. Think of a block of ice or a piece of metal – their shape and volume remain constant unless subjected to external forces that overcome the strong intermolecular interactions.
Liquids: Fixed Volume, Variable Shape
Liquids, on the other hand, have a fixed volume but a variable shape. While their particles are still relatively close together, they have more freedom of movement than those in a solid. The intermolecular forces are weaker, allowing the particles to slide past one another, enabling the liquid to conform to the shape of its container. Consider water poured into a glass – it takes on the shape of the glass, but its volume remains the same.
Gases: Variable Volume and Shape
Gases stand in stark contrast to solids and liquids. They have neither a fixed volume nor a fixed shape. The particles in a gas are widely dispersed and move independently at high speeds. The weak intermolecular forces allow the particles to move freely, resulting in a gas expanding to fill whatever container it occupies. A balloon filled with air, for example, takes on the shape and volume of the balloon itself, demonstrating the adaptability of gas to its environment.
The Kinetic Molecular Theory of Gases: Explaining Variable Volume
The kinetic molecular theory of gases provides a powerful model for understanding the behavior of gases, including their variable volume. This theory is based on several key postulates:
- Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. This constant movement is a source of the gas's kinetic energy.
- The volume of the gas particles themselves is negligible compared to the volume of the container. This means the particles occupy a tiny fraction of the total space.
- There are no attractive or repulsive forces between the gas particles. This assumption simplifies the model and works well for ideal gases.
- Collisions between gas particles and the container walls are elastic. This implies that no kinetic energy is lost during collisions.
- The average kinetic energy of the gas particles is directly proportional to the absolute temperature of the gas. This link between kinetic energy and temperature is crucial.
This theory clearly explains why gases don't have a fixed volume. Because the particles are in constant, random motion and there are negligible interparticle forces, the gas expands to fill the available space. The volume of the gas is thus determined by the size of its container.
Factors Affecting Gas Volume: Pressure and Temperature
Two crucial factors determine the volume of a gas: pressure and temperature. These are intricately linked and described through the ideal gas law, a cornerstone of chemistry and physics.
Pressure and its Influence
Pressure is defined as the force exerted per unit area. In a gas, pressure arises from the collisions of gas particles with the walls of the container. Increasing the pressure on a gas forces the particles closer together, resulting in a decrease in volume. Conversely, decreasing the pressure allows the particles to spread out, leading to an increase in volume. This relationship is inversely proportional, as articulated in Boyle's Law: at constant temperature, the volume of a gas is inversely proportional to its pressure (V ∝ 1/P).
Temperature and its Effects
Temperature is a measure of the average kinetic energy of the gas particles. Increasing the temperature increases the kinetic energy of the particles, causing them to move faster and collide more forcefully with the container walls. This leads to an expansion in volume. Conversely, decreasing the temperature slows down the particles, reducing their kinetic energy and resulting in a decrease in volume. This relationship is directly proportional, as stated in Charles's Law: at constant pressure, the volume of a gas is directly proportional to its absolute temperature (V ∝ T).
The Ideal Gas Law: A Comprehensive Relationship
The ideal gas law combines Boyle's Law and Charles's Law, along with Avogadro's Law (which states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules), into a single, powerful equation:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of gas
- R is the ideal gas constant
- T is the absolute temperature of the gas
This equation shows the intricate interdependence of pressure, volume, temperature, and the amount of gas. It's important to note that the ideal gas law is an approximation; real gases deviate from ideal behavior at high pressures and low temperatures.
Real Gases vs. Ideal Gases: Deviations from Ideal Behavior
The ideal gas law works remarkably well for many gases under ordinary conditions. However, real gases deviate from ideal behavior under certain conditions, primarily due to two factors:
- Intermolecular forces: Ideal gases assume no intermolecular forces. In reality, attractive forces exist between gas particles, especially at low temperatures and high pressures. These forces reduce the effective pressure and volume, causing deviations from the ideal gas law.
- Finite particle volume: The ideal gas law assumes that the volume of gas particles is negligible. However, at high pressures, the volume occupied by the particles themselves becomes significant, leading to deviations.
To account for these deviations, more complex equations of state, such as the van der Waals equation, have been developed. These equations incorporate correction factors to address the intermolecular forces and finite particle volumes.
Applications of Understanding Gas Volume
The understanding of gas volume and its relationship to pressure and temperature has far-reaching applications in various fields:
- Meteorology: Weather forecasting heavily relies on understanding how atmospheric pressure and temperature affect air volume and movement.
- Automotive engineering: Internal combustion engines depend on the controlled expansion and compression of gases to generate power.
- Aerospace engineering: Designing aircraft and spacecraft involves precise calculations of gas behavior at varying altitudes and temperatures.
- Chemical engineering: Numerous industrial processes involve the handling and manipulation of gases, requiring a thorough understanding of their properties.
- Medical applications: Respiratory therapy and anesthesia utilize controlled gas delivery and pressure management.
Conclusion: Gas Volume is Dynamic, Not Fixed
In conclusion, gas does not have a fixed volume. Its volume is a dynamic property, highly dependent on pressure and temperature, and accurately described by the ideal gas law (and more complex equations for real gases). This understanding is foundational to numerous scientific principles and has widespread practical applications across various industries. The constant motion of gas particles, the weak intermolecular forces, and the direct correlation with pressure and temperature are the key reasons why gas volume is flexible and adaptable to its surroundings. The ability to predict and control gas volume is essential in countless areas of science and technology.
Latest Posts
Latest Posts
-
Describe The Sampling Distribution Of P Hat
Mar 25, 2025
-
How To Determine State Of Matter In Chemical Equation
Mar 25, 2025
-
Resistors In Series And Parallel Calculator
Mar 25, 2025
-
Where Are Breathing Control Centers Located
Mar 25, 2025
-
Most Digestion And Absorption Of Food Occurs In The
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Does Gas Have A Fixed Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
