Does Reaction Rate Depend On Concentration Of The Catalyst
Muz Play
Mar 16, 2025 · 5 min read
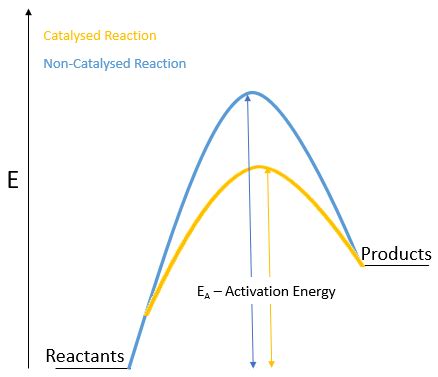
Table of Contents
Does Reaction Rate Depend on the Concentration of the Catalyst? A Deep Dive into Catalysis
The speed at which a chemical reaction proceeds, its reaction rate, is a crucial factor in many chemical processes, from industrial manufacturing to biological systems. Numerous factors influence this rate, including temperature, pressure, surface area, and crucially, the presence of a catalyst. This article delves into the intricate relationship between reaction rate and catalyst concentration, exploring the mechanisms behind this dependency and its implications across various chemical domains.
Understanding Catalysts and Their Role
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. It achieves this by providing an alternative reaction pathway with a lower activation energy, the minimum energy required for reactants to transform into products. This lower energy barrier allows more reactant molecules to overcome it at a given temperature, thereby accelerating the reaction.
Catalysts function through various mechanisms, often involving the formation of intermediate complexes between the catalyst and reactants. These complexes destabilize the reactants, making them more susceptible to transformation into products. The catalyst is then regenerated in the process, leaving its overall quantity unchanged.
The Concentration-Rate Relationship: A Non-Linear Affair
The impact of catalyst concentration on reaction rate is not always straightforward. While generally, increasing catalyst concentration increases the reaction rate, the relationship is rarely linear. The exact nature of this relationship depends on several factors, including:
- The type of catalysis: Homogeneous catalysis (catalyst in the same phase as reactants) often shows a more direct relationship between concentration and rate than heterogeneous catalysis (catalyst in a different phase).
- The reaction mechanism: The specific steps involved in the catalyzed reaction determine how catalyst concentration affects the rate. Some mechanisms exhibit saturation kinetics, where increasing catalyst concentration beyond a certain point yields diminishing returns.
- The presence of inhibitors or poisons: Substances that interfere with the catalyst's function can significantly alter the concentration-rate relationship.
Homogeneous Catalysis: A Simpler Picture
In homogeneous catalysis, the catalyst is uniformly distributed within the reaction mixture. The rate often exhibits a direct proportionality to the catalyst concentration, at least within a certain range. Consider a simple reaction:
A + B → C
If a catalyst, denoted by 'Cat', facilitates this reaction through a mechanism involving the formation of an intermediate complex, the rate law might appear as:
Rate = k [A][B][Cat]
where 'k' is the rate constant. This equation clearly demonstrates the direct proportionality between the reaction rate and the catalyst concentration. However, this idealized model often needs refinement to account for more complex scenarios.
Heterogeneous Catalysis: A More Complex Scenario
Heterogeneous catalysis, where the catalyst exists in a different phase (e.g., a solid catalyst in a liquid reaction mixture), presents a more nuanced picture. The reaction rate isn't solely determined by the bulk concentration of the catalyst but also by:
- The surface area of the catalyst: Only molecules interacting with the catalyst's surface can participate in the reaction. Therefore, a highly porous catalyst with a large surface area will exhibit a greater rate enhancement compared to a less porous catalyst with the same mass.
- The adsorption and desorption processes: Reactants must adsorb (bind) onto the catalyst's surface, react, and then desorb (detach) from the surface. These steps can be rate-limiting, and their kinetics will influence the overall reaction rate. At high concentrations, surface saturation can occur, limiting further rate enhancement.
- Catalyst deactivation: Heterogeneous catalysts are susceptible to deactivation, where their activity decreases over time due to poisoning, sintering (agglomeration of catalyst particles), or fouling (accumulation of impurities on the surface). This can lead to a non-linear or even decreasing relationship between catalyst concentration and rate at higher concentrations.
Saturation Kinetics: The Limits of Rate Enhancement
In many catalytic reactions, especially those involving heterogeneous catalysis or enzyme-catalyzed reactions, the relationship between reaction rate and catalyst concentration follows saturation kinetics. This means that as catalyst concentration increases, the reaction rate initially increases rapidly, but eventually, the rate increase slows down and plateaus. This plateau occurs because the available catalyst surface area or active sites become saturated with reactants. Adding more catalyst beyond this saturation point will have minimal effect on the rate.
The Michaelis-Menten equation, commonly used in enzyme kinetics, describes saturation kinetics:
Rate = (Vmax * [S]) / (Km + [S])
Where:
- Vmax is the maximum reaction rate
- [S] is the substrate (reactant) concentration
- Km is the Michaelis constant, representing the substrate concentration at half the maximum reaction rate.
This equation highlights that even with an infinite amount of catalyst (or enzyme), the reaction rate cannot exceed Vmax.
Practical Implications and Examples
The understanding of how catalyst concentration affects reaction rate is crucial in various applications:
- Industrial catalysis: Optimizing catalyst loading in industrial reactors is essential for maximizing efficiency and minimizing costs. Too little catalyst leads to slow reactions, while excessive amounts might lead to diminishing returns and waste of resources.
- Environmental catalysis: Designing efficient catalytic converters for automobiles or industrial emission control requires careful consideration of catalyst concentration and surface area to ensure effective pollutant removal.
- Enzyme catalysis: Understanding enzyme kinetics in biological systems is essential for comprehending metabolic pathways and developing pharmaceuticals.
Example: The Haber-Bosch process, used for ammonia synthesis, employs an iron-based catalyst. The efficiency of ammonia production is highly dependent on the catalyst's properties, including its surface area and concentration, to ensure optimal reaction rates. Similarly, the catalytic converters in automobiles rely on precisely engineered catalyst compositions to effectively convert harmful exhaust gases into less harmful substances.
Conclusion: A Dynamic Interplay
The relationship between reaction rate and catalyst concentration is complex and depends on various factors. While a general trend of increased rate with increased concentration exists, saturation kinetics and other limiting factors can lead to non-linear behavior. A thorough understanding of the specific reaction mechanism, the type of catalysis, and potential deactivation processes is crucial for predicting and optimizing the impact of catalyst concentration on the reaction rate. This understanding is fundamental for designing efficient and sustainable chemical processes across various industrial, environmental, and biological applications. Further research continues to refine our knowledge of this crucial relationship, leading to advancements in catalyst design and reaction engineering.
Latest Posts
Latest Posts
-
Effective Nuclear Charge Vs Nuclear Charge
Mar 17, 2025
-
What Is The Opposite Of Sublimation
Mar 17, 2025
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Reaction Rate Depend On Concentration Of The Catalyst . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
