Electrically Neutral Atoms Have Equal Numbers Of Electrons And Protons
Muz Play
Mar 20, 2025 · 7 min read
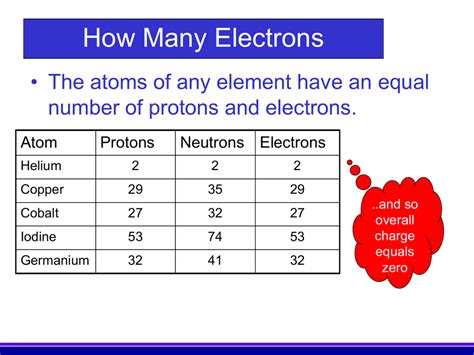
Table of Contents
Electrically Neutral Atoms: A Deep Dive into the Balance of Electrons and Protons
The fundamental principle governing the stability and behavior of atoms is the balance between their constituent particles: protons and electrons. This article delves into the crucial concept of electrical neutrality in atoms, exploring why electrically neutral atoms possess an equal number of electrons and protons, and the implications of this balance for atomic structure, chemical bonding, and the overall properties of matter.
The Building Blocks of Matter: Protons, Electrons, and Neutrons
Before diving into the specifics of electrical neutrality, let's revisit the basic components of an atom. Atoms are the fundamental units of matter, composed of three primary subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons determines the atom's atomic number and its identity as a specific element. For example, hydrogen has one proton, carbon has six, and oxygen has eight.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. Electrons are significantly lighter than protons.
-
Neutrons: Neutral particles (no charge) also located in the atom's nucleus. Neutrons contribute to the atom's mass but not its charge. The number of neutrons can vary within the same element, leading to isotopes.
The Significance of Electrical Charge
Electrical charge is a fundamental property of matter, manifesting as attraction or repulsion between particles. Like charges (positive-positive or negative-negative) repel each other, while opposite charges (positive-negative) attract. This electrostatic force plays a crucial role in holding atoms together and influencing their interactions.
The magnitude of the charge carried by a proton is equal and opposite to that of an electron. This means that one proton's positive charge perfectly cancels out one electron's negative charge.
Electrical Neutrality: The Foundation of Atomic Stability
An atom is considered electrically neutral when the number of positively charged protons in its nucleus precisely equals the number of negatively charged electrons orbiting the nucleus. This balance of charges results in a net charge of zero.
Why is electrical neutrality so important?
The significance of electrical neutrality stems from the strong electrostatic forces between protons and electrons. If an atom had an unequal number of protons and electrons, it would carry a net positive (more protons) or negative (more electrons) charge, becoming an ion. Ions are highly reactive and readily participate in chemical reactions to achieve stability, often by gaining or losing electrons to reach a neutral state.
In contrast, electrically neutral atoms are generally more stable and less reactive. The attractive forces between the positively charged nucleus and the negatively charged electrons hold the atom together, creating a relatively stable and balanced system. This stability is crucial for the formation of molecules and the existence of matter as we know it.
Understanding Electron Shells and Energy Levels
Electrons don't simply orbit the nucleus randomly. They occupy specific energy levels or shells, each with a defined capacity for electrons. The first shell (closest to the nucleus) can hold a maximum of two electrons, the second shell can hold up to eight, and so on. The arrangement of electrons in these shells dictates an atom's chemical properties and its ability to form bonds with other atoms.
The filling of electron shells follows specific rules, primarily the Aufbau principle, Hund's rule, and the Pauli exclusion principle. These rules determine the electron configuration of an atom, which is a crucial factor in determining its reactivity and chemical behavior.
The Aufbau Principle
The Aufbau principle states that electrons fill the lowest available energy levels first. This means electrons will fill the inner shells before moving to outer shells.
Hund's Rule
Hund's rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion.
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This essentially limits the number of electrons that can occupy a specific orbital.
Ions: When the Balance is Disturbed
While electrically neutral atoms are the norm, it's important to acknowledge the existence of ions. Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge.
-
Cations: Positively charged ions formed when an atom loses electrons. For example, a sodium atom (Na) can lose one electron to become a sodium ion (Na⁺).
-
Anions: Negatively charged ions formed when an atom gains electrons. For example, a chlorine atom (Cl) can gain one electron to become a chloride ion (Cl⁻).
Ion formation is a common process in chemical reactions, and it's driven by the tendency of atoms to achieve a stable electron configuration, often resembling the electron configuration of a noble gas (Group 18 elements). The formation of ions plays a critical role in ionic bonding, a type of chemical bond formed by the electrostatic attraction between oppositely charged ions.
Implications of Electrical Neutrality for Chemical Bonding
Electrical neutrality is fundamental to understanding chemical bonding. The interaction between atoms and the formation of molecules are primarily governed by the distribution and behavior of electrons. Three primary types of chemical bonds illustrate this connection:
-
Ionic Bonds: Ionic bonds are formed through the electrostatic attraction between oppositely charged ions (cations and anions). This type of bond arises when one atom transfers one or more electrons to another atom, leading to the formation of ions. The resulting electrostatic attraction holds the ions together. Examples include sodium chloride (NaCl) and magnesium oxide (MgO).
-
Covalent Bonds: Covalent bonds involve the sharing of electrons between atoms. In this type of bond, atoms achieve a more stable electron configuration by sharing electrons, rather than transferring them completely. Covalent bonds are prevalent in organic molecules and many other compounds. Examples include water (H₂O) and methane (CH₄).
-
Metallic Bonds: Metallic bonds occur in metals, where valence electrons are delocalized and shared among a "sea" of electrons. This electron sea allows for high electrical conductivity and malleability.
The Role of Isotopes and Nuclear Stability
While the number of protons defines an element, the number of neutrons can vary. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. While isotopes have different masses, they retain electrical neutrality as long as the number of electrons equals the number of protons.
However, the balance of protons and neutrons in the nucleus also influences nuclear stability. Some isotopes are radioactive, meaning their nuclei are unstable and undergo radioactive decay, emitting particles or energy to reach a more stable state. This decay can alter the number of protons and neutrons in the nucleus, but the atom strives to maintain charge neutrality by adjusting its electron count.
Advanced Concepts: Ionization Energy and Electron Affinity
Two important concepts related to electrical neutrality and atomic structure are ionization energy and electron affinity:
-
Ionization Energy: This is the energy required to remove an electron from a neutral atom in its gaseous state. It's a measure of how strongly an atom holds onto its electrons. Higher ionization energies indicate a greater tendency for an atom to remain neutral.
-
Electron Affinity: This is the energy change associated with adding an electron to a neutral atom in its gaseous state. A high electron affinity indicates a strong tendency for an atom to gain an electron and become a negatively charged ion.
These properties are essential for understanding chemical reactivity and the formation of chemical bonds. They directly reflect the balance between the attractive forces of the nucleus and the repulsive forces between electrons.
Conclusion: The Cornerstone of Chemistry and Physics
The principle of electrically neutral atoms having equal numbers of electrons and protons is a cornerstone of our understanding of chemistry and physics. This fundamental balance underpins atomic structure, chemical bonding, and the properties of matter. While ions exist and play important roles, the tendency towards neutrality dictates the stability and reactivity of atoms and molecules, shaping the world around us. Further exploration of this principle leads to a deeper understanding of the complex interactions within matter, paving the way for advances in various scientific fields. From the development of new materials to the unraveling of complex biological processes, the principle of electrical neutrality remains a crucial concept in scientific inquiry.
Latest Posts
Latest Posts
-
The Basic Unit Of Life Is The Cell
Mar 21, 2025
-
Label The Arteries Of The Head And Neck
Mar 21, 2025
-
Hardness Is A Physical Or Chemical Property
Mar 21, 2025
-
What Is A Spectrophotometer Used For
Mar 21, 2025
-
Area Of Non Right Angle Triangle
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Electrically Neutral Atoms Have Equal Numbers Of Electrons And Protons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
