Electrolysis Is When Chemicals Break Down Into Charged Particles Called
Muz Play
Mar 29, 2025 · 6 min read
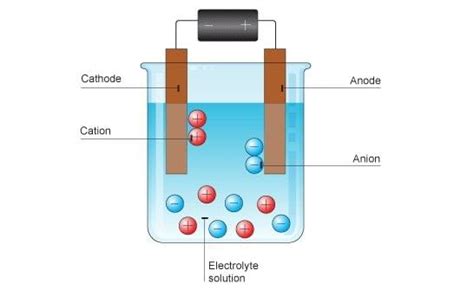
Table of Contents
Electrolysis: When Chemicals Break Down into Charged Particles Called Ions
Electrolysis is a powerful electrochemical process with wide-ranging applications in various industries. At its core, electrolysis involves the breakdown of a chemical compound into its constituent ions using an electric current. Understanding this fundamental process requires a grasp of key concepts such as electrolytes, electrodes, and the role of electricity in driving chemical reactions. This comprehensive guide delves deep into the intricacies of electrolysis, explaining its mechanisms, applications, and significance in both scientific research and industrial processes.
Understanding the Basics of Electrolysis
Electrolysis, derived from the Greek words "electro" (electricity) and "lysis" (to break down), is fundamentally the decomposition of a substance into its simpler components using direct electric current. This process is driven by an external source of electrical energy, which forces electrons to flow through an electrolytic solution or molten electrolyte. This flow of electrons facilitates the oxidation and reduction reactions at the electrodes, leading to the liberation of ions.
Electrolytes: The Medium for Ionization
The heart of any electrolytic process lies in the electrolyte, which is a substance that conducts electricity when dissolved in a solvent or molten. Electrolytes are typically ionic compounds, which means they readily dissociate into positively charged cations and negatively charged anions when subjected to sufficient energy (heat or electricity). Common electrolytes include aqueous solutions of salts, acids, and bases, as well as molten ionic compounds. The ability of an electrolyte to conduct electricity is directly proportional to its concentration of ions – the higher the concentration, the greater the conductivity.
Electrodes: The Gateway for Electron Transfer
Electrodes are crucial components in an electrolytic cell. They act as conduits for the electrons flowing from the external power source into the electrolyte and vice-versa. There are two types of electrodes:
-
Anode: This is the positive electrode where oxidation occurs. Oxidation is the process where a substance loses electrons, resulting in an increase in its oxidation state. At the anode, anions (negatively charged ions) lose electrons and are oxidized.
-
Cathode: This is the negative electrode where reduction occurs. Reduction is the process where a substance gains electrons, resulting in a decrease in its oxidation state. At the cathode, cations (positively charged ions) gain electrons and are reduced.
The materials used for electrodes are chosen based on their chemical inertness and conductivity. Common electrode materials include platinum, graphite, and various metals. The choice of electrode material can significantly influence the efficiency and selectivity of the electrolytic process.
The Electrolytic Process: A Step-by-Step Breakdown
The electrolysis process involves several interconnected steps:
-
Ionization: When the electrolyte is dissolved or melted, it dissociates into its constituent ions. For example, in an aqueous solution of sodium chloride (NaCl), it dissociates into Na⁺ (sodium cations) and Cl⁻ (chloride anions).
-
Migration of Ions: Under the influence of the applied electric field, the ions migrate towards the electrodes of opposite charge. Cations move towards the cathode (negatively charged), while anions move towards the anode (positively charged).
-
Electron Transfer: At the electrodes, electron transfer occurs. At the anode, anions lose electrons (oxidation), and at the cathode, cations gain electrons (reduction).
-
Chemical Reactions: The electron transfer reactions at the electrodes lead to chemical changes. For instance, in the electrolysis of NaCl, chloride ions (Cl⁻) lose electrons at the anode to form chlorine gas (Cl₂), and sodium ions (Na⁺) gain electrons at the cathode to form sodium metal (Na).
-
Product Formation: The chemical reactions at the electrodes result in the formation of new substances – these are the products of electrolysis. In our example, the products are chlorine gas and sodium metal.
Faraday's Laws of Electrolysis
Michael Faraday's laws elegantly quantify the relationship between the amount of substance decomposed or produced during electrolysis and the quantity of electricity passed through the electrolytic cell. These laws are fundamental to understanding the quantitative aspects of electrolysis:
-
Faraday's First Law: The mass of a substance deposited or liberated at an electrode is directly proportional to the quantity of electricity passed through the electrolyte. This means that doubling the amount of charge passed through the cell will double the mass of the substance deposited.
-
Faraday's Second Law: The masses of different substances deposited or liberated by the same quantity of electricity are proportional to their equivalent weights. The equivalent weight is the molar mass of a substance divided by its charge.
These laws are crucial for calculating the amount of substance produced or consumed in an electrolytic process, given the current and time of electrolysis.
Applications of Electrolysis: A Diverse Range of Industries
Electrolysis has far-reaching applications across numerous industries, impacting our daily lives in profound ways. Some key applications include:
1. Metal Extraction and Refining:
Electrolysis is a cornerstone of the metallurgical industry, used to extract and purify various metals. Examples include:
- Aluminum production: The Hall-Héroult process uses electrolysis to extract aluminum from its ore, bauxite.
- Copper refining: Electrorefining utilizes electrolysis to purify copper, removing impurities and increasing its conductivity.
- Extraction of alkali and alkaline earth metals: Electrolysis is used to extract highly reactive metals like sodium, potassium, magnesium, and calcium from their molten salts.
2. Electroplating and Electroforming:
Electroplating is a widely used technique to deposit a thin layer of metal onto another surface. This process enhances the appearance, corrosion resistance, and wear resistance of the underlying material. Electroforming utilizes electrolysis to create complex three-dimensional shapes by depositing metal onto a mold.
3. Chlor-Alkali Process:
This industrial process uses electrolysis to produce chlorine gas (Cl₂), sodium hydroxide (NaOH), and hydrogen gas (H₂). These products are essential in various industrial applications, including water treatment, plastics manufacturing, and paper production.
4. Water Purification:
Electrolysis can purify water by removing dissolved impurities and contaminants. Electrocoagulation and electrooxidation processes effectively remove heavy metals, organic pollutants, and other undesirable substances from water.
5. Fuel Cell Technology:
Electrolysis plays a vital role in fuel cell technology, specifically in the production of hydrogen gas, a clean and sustainable fuel source. Electrolysis of water produces hydrogen and oxygen, which can then be used in fuel cells to generate electricity.
6. Batteries and Energy Storage:
Electrolysis is involved in the charging process of rechargeable batteries, where the chemical reactions within the battery are reversed to replenish the stored energy.
Beyond the Basics: Advanced Electrolytic Techniques
While the fundamental principles of electrolysis remain consistent, advancements in technology have led to the development of sophisticated electrolytic techniques:
Pulse Electrolysis:
This technique uses short bursts of electric current instead of continuous current, leading to improved efficiency and selectivity in certain processes.
Ultrasonically Assisted Electrolysis:
Combining ultrasound with electrolysis enhances mass transport and reaction kinetics, improving the efficiency of the electrolytic process.
Microfluidic Electrolysis:
This miniaturized approach offers precise control over reaction parameters and allows for high-throughput analysis.
Conclusion: The Enduring Importance of Electrolysis
Electrolysis, despite its seemingly simple principles, is a powerful and versatile electrochemical process with far-reaching consequences in diverse scientific and industrial domains. From metal extraction to water purification, its contributions to modern technology are undeniable. Further research and advancements in electrolytic techniques are poised to open even more exciting possibilities, promising greener and more efficient processes for a sustainable future. The understanding of electrolysis, therefore, extends beyond mere academic interest; it provides a foundational understanding of fundamental chemistry and its impact on our world. As technology continues to evolve, the applications of electrolysis will undoubtedly continue to expand, shaping our future in remarkable ways.
Latest Posts
Latest Posts
-
Do Acids And Bases Conduct Electricity
Mar 31, 2025
-
Inverse Relations And Functions Quick Check
Mar 31, 2025
-
Table Salt Is A Pure Substance
Mar 31, 2025
-
What Makes Sour Patch Kids Sour
Mar 31, 2025
-
Is Urea The Same As Uric Acid
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Electrolysis Is When Chemicals Break Down Into Charged Particles Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
