Electrophilic Addition Of Hbr To An Alkene
Muz Play
Mar 20, 2025 · 5 min read
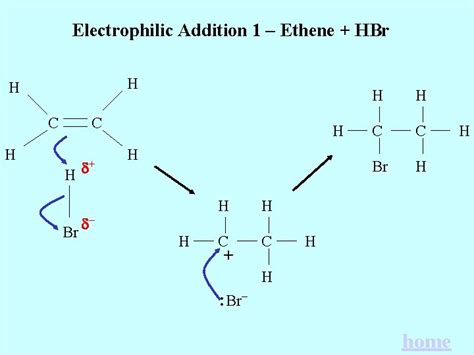
Table of Contents
Electrophilic Addition of HBr to Alkenes: A Deep Dive
The electrophilic addition of hydrogen bromide (HBr) to alkenes is a fundamental reaction in organic chemistry, serving as a cornerstone for understanding reaction mechanisms and synthetic applications. This reaction, governed by Markovnikov's rule, provides a straightforward method for the synthesis of alkyl halides, crucial building blocks in various organic syntheses. This comprehensive guide will explore the mechanism, regioselectivity, stereochemistry, and synthetic applications of this important transformation.
Understanding the Reaction Mechanism
The electrophilic addition of HBr to an alkene proceeds through a two-step mechanism involving a carbocation intermediate. Let's break it down:
Step 1: Electrophilic Attack
The reaction initiates with the electrophilic attack of the hydrogen atom (δ+) of HBr on the alkene's π-bond. The alkene's electron-rich double bond acts as a nucleophile, attacking the partially positive hydrogen. This step results in the formation of a carbocation, a positively charged carbon atom, and a bromide ion (Br⁻). The positive charge resides on the carbon atom that now bears only three bonds.
Key Point: The stability of the carbocation intermediate dictates the regioselectivity of the reaction (more on this later). More substituted carbocations (tertiary > secondary > primary) are more stable due to hyperconjugation and inductive effects.
Step 2: Nucleophilic Attack
In the second step, the bromide ion (Br⁻), a nucleophile, attacks the carbocation. This nucleophilic attack forms a new carbon-bromine bond, resulting in the final product, an alkyl halide.
Visual Representation:
Imagine the alkene's double bond as two electrons loosely held between two carbon atoms. The partially positive hydrogen in HBr attracts these electrons, breaking the pi bond and forming a new sigma bond to one of the carbons. This leaves the other carbon with a positive charge (the carbocation). The bromide ion then bonds to this positively charged carbon, neutralizing the charge and forming the alkyl halide.
Markovnikov's Rule: Dictating Regioselectivity
The addition of HBr to an unsymmetrical alkene follows Markovnikov's rule. This rule states that the hydrogen atom adds to the carbon atom that already has the greater number of hydrogen atoms. Consequently, the bromine atom adds to the carbon atom with fewer hydrogen atoms – the more substituted carbon.
Why Markovnikov's Rule?
Markovnikov's rule is a consequence of the stability of the carbocation intermediate. The more substituted carbocation formed during the initial electrophilic attack is more stable. The reaction preferentially proceeds through the more stable intermediate, leading to the major product predicted by Markovnikov's rule.
Stereochemistry of the Reaction
The addition of HBr to an alkene is generally not stereospecific. This means that the reaction does not produce a single stereoisomer. If the alkene is not symmetrical, the product will be a racemic mixture if a chiral center is formed. This lack of stereospecificity is because the carbocation intermediate is planar, allowing nucleophilic attack from either side with equal probability.
Factors Influencing the Reaction
Several factors can influence the reaction rate and product distribution:
- Solvent: Polar protic solvents can stabilize the carbocation intermediate and increase the reaction rate.
- Temperature: Higher temperatures generally increase the reaction rate but may also lead to side reactions.
- Concentration of reactants: The concentration of HBr and the alkene affects the reaction rate.
- Presence of peroxides: This is crucial! The presence of peroxides can lead to anti-Markovnikov addition, a topic explored in the next section.
Anti-Markovnikov Addition: The Role of Peroxides
In the presence of peroxides (e.g., dibenzoyl peroxide), the addition of HBr to alkenes proceeds via a free radical mechanism, leading to anti-Markovnikov addition. This is a significant exception to Markovnikov's rule.
Free Radical Mechanism
The free radical mechanism involves three steps:
-
Initiation: Peroxides decompose to generate free radicals, which then abstract a hydrogen atom from HBr, forming a bromine radical (Br•).
-
Propagation: The bromine radical attacks the alkene, forming a more substituted carbon radical. This is unlike the carbocation mechanism, where stability favors the more substituted carbocation. This carbon radical then abstracts a hydrogen from another molecule of HBr, resulting in the formation of the anti-Markovnikov product and a new bromine radical, perpetuating the chain reaction.
-
Termination: The chain reaction terminates when two radicals combine.
Key Difference: The key difference between the ionic and free radical mechanisms lies in the intermediate formed. The ionic mechanism involves a carbocation, while the free radical mechanism involves a carbon radical. The stability of these intermediates dictates the regioselectivity of the reaction.
Synthetic Applications
The electrophilic addition of HBr to alkenes is a versatile reaction with numerous synthetic applications. It's a crucial step in the synthesis of various compounds, including:
-
Alkyl halides: As mentioned earlier, alkyl halides are important building blocks for many organic synthesis reactions. They can undergo substitution and elimination reactions to generate a wide array of functional groups.
-
Grignard reagents: Alkyl halides can be converted into Grignard reagents, powerful nucleophiles used in various carbon-carbon bond-forming reactions.
-
Alcohols: Alkyl halides can be further converted into alcohols through nucleophilic substitution or reduction reactions.
-
Amines: Alkyl halides are used in the synthesis of amines via nucleophilic substitution with ammonia or primary/secondary amines.
-
Ethers: Williamson ether synthesis, employing alkyl halides, is a prevalent method for ether synthesis.
Conclusion
The electrophilic addition of HBr to alkenes is a fundamental reaction with a rich mechanistic detail and broad synthetic utility. Understanding the nuances of the reaction, including Markovnikov's rule, the influence of peroxides leading to anti-Markovnikov addition, and the stereochemical aspects, is essential for any aspiring organic chemist. Its applications span a wide range of organic synthesis, cementing its importance in the field. By mastering this reaction, one gains a firm foundation in understanding reaction mechanisms and designing effective synthetic strategies. This knowledge allows for the strategic planning and execution of complex organic syntheses, paving the way for the discovery and development of new molecules with diverse functionalities and applications. Furthermore, the ability to predict and control the regioselectivity and stereochemistry of this reaction is crucial for optimizing yields and obtaining desired products. The continued study and refinement of this classic reaction will undoubtedly contribute to advancements in various chemical disciplines.
Latest Posts
Latest Posts
-
An Organism That Obtains Energy By Eating Other Organisms
Mar 20, 2025
-
Label The Parts Of The Phospholipid
Mar 20, 2025
-
Why Is Chemistry Important In The Study Of Biology
Mar 20, 2025
-
How To Determine The Equivalence Point On A Titration Curve
Mar 20, 2025
-
What Does A Flat Slope Indicate
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Electrophilic Addition Of Hbr To An Alkene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
