How To Determine The Equivalence Point On A Titration Curve
Muz Play
Mar 20, 2025 · 6 min read
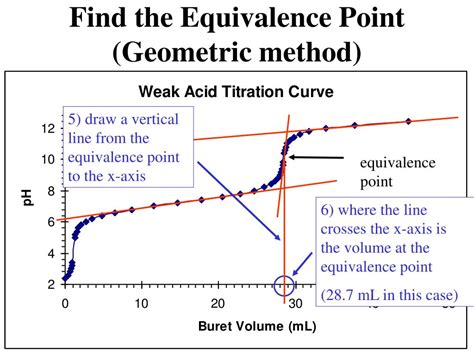
Table of Contents
How to Determine the Equivalence Point on a Titration Curve
Titration is a fundamental analytical technique used extensively in chemistry to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). The process involves carefully adding the titrant to the analyte until the reaction is complete, a point known as the equivalence point. Identifying this equivalence point accurately is crucial for obtaining reliable results. This article will delve into the methods used to determine the equivalence point from a titration curve, emphasizing both visual and calculated approaches.
Understanding the Titration Curve
Before diving into the methods, let's understand what a titration curve represents. A titration curve is a graphical representation of the change in pH (or other suitable property) of the analyte solution as a function of the volume of titrant added. The curve typically shows a gradual change in pH initially, followed by a sharp, almost vertical change near the equivalence point, and then a gradual change again. The shape of the curve depends on the specific acid-base reaction involved (strong acid-strong base, weak acid-strong base, etc.).
Key Features of a Titration Curve
- Initial pH: The pH of the analyte solution before any titrant is added. This is determined by the concentration and strength of the analyte.
- Buffer Region: A region where the pH changes relatively slowly. This is often observed in titrations involving weak acids or bases.
- Equivalence Point: The point at which the moles of titrant added are stoichiometrically equivalent to the moles of analyte present. This is the point we are trying to determine.
- Half-Equivalence Point: The point at which half the volume of titrant needed to reach the equivalence point has been added. For weak acid-strong base titrations, the pH at this point is equal to the pKa of the weak acid.
- End Point: The point at which a significant change in pH (or other property) is observed, usually indicated by a color change in an indicator. Ideally, the end point should coincide with the equivalence point, but there is often a slight difference.
Methods for Determining the Equivalence Point
Several methods can be used to determine the equivalence point from a titration curve. These methods can be broadly categorized as visual and calculated methods.
Visual Methods: Using Indicators
Traditionally, the equivalence point was estimated visually using indicators. Indicators are substances that change color depending on the pH of the solution. The choice of indicator depends on the pH range of the equivalence point. For example, phenolphthalein is a common indicator for strong acid-strong base titrations because it changes color around pH 8-10, which is close to the equivalence point for such titrations. However, this method is inherently less precise than calculated methods. The visual detection of the color change is subjective and dependent on the observer's perception.
Calculated Methods: Analyzing the Titration Curve Data
More accurate determination of the equivalence point relies on analyzing the titration curve data obtained through a pH meter. Several calculated methods can be employed:
1. First Derivative Method
This method involves calculating the first derivative of the titration curve (ΔpH/ΔV). The equivalence point corresponds to the point of maximum slope on the curve, or the point where the first derivative is at its maximum value. This method is relatively straightforward but can be sensitive to experimental error, especially if the curve doesn't exhibit a sharp inflection point.
How to calculate:
- Tabulate your data: Create a table with columns for volume (V) of titrant added and the corresponding pH.
- Calculate the ΔpH/ΔV: For each data point, calculate the change in pH (ΔpH) divided by the change in volume (ΔV) between consecutive data points.
- Plot the first derivative: Plot the calculated ΔpH/ΔV values against the average volume (V). The maximum value of this plot indicates the equivalence point.
2. Second Derivative Method
The second derivative method is a refinement of the first derivative method. It involves calculating the second derivative of the titration curve (Δ(ΔpH/ΔV)/ΔV). The equivalence point corresponds to the point where the second derivative is zero, indicating a change in the direction of the slope of the first derivative curve. This method is generally less sensitive to minor irregularities in the titration curve data compared to the first derivative method.
How to calculate:
- Obtain the first derivative: Follow the steps in the first derivative method to obtain the first derivative values.
- Calculate the second derivative: For each consecutive pair of first derivative values, calculate the change in the first derivative divided by the change in volume (Δ(ΔpH/ΔV)/ΔV).
- Plot the second derivative: Plot the calculated second derivative values against the average volume (V). The point where the second derivative crosses zero represents the equivalence point.
3. Gran Plot Method
The Gran plot method is particularly useful for titrations involving weak acids or bases where the equivalence point is not sharply defined. It involves plotting a function of the pH and volume of titrant added. The equivalence point is determined from the x-intercept of the linear region of the plot. Different Gran plots are used for different titration types (e.g., strong acid-strong base, weak acid-strong base).
How to create a Gran plot:
- Select the appropriate Gran plot equation. For a strong acid-strong base titration: V<sub>b</sub> × 10<sup>-pH</sup> (where V<sub>b</sub> is the volume of base added).
- Calculate the function: For each data point, calculate the value of the chosen function.
- Plot the data: Plot the calculated function values against the volume of titrant added (V). The x-intercept of the linear portion of the plot corresponds to the equivalence point.
4. Software and Numerical Methods
Many sophisticated software packages are available that can analyze titration curve data and determine the equivalence point using various numerical methods, including least-squares fitting and other algorithms. These methods can be more accurate and robust than manual calculations, particularly for complex titration curves. These softwares often offer multiple methods for equivalence point determination, allowing for cross-validation of results.
Choosing the Best Method
The optimal method for determining the equivalence point depends on several factors:
- Type of titration: The choice of method is influenced by whether it's a strong acid-strong base, weak acid-strong base, or other types of titrations. Gran plots are particularly useful for weak acid-weak base titrations.
- Accuracy required: For high accuracy, calculated methods are preferred over visual methods.
- Data quality: The accuracy of the calculated methods depends on the quality of the experimental data. Accurate pH measurements are crucial.
- Availability of software: The use of software packages can simplify the process and improve accuracy, especially for complex curves.
Conclusion
Determining the equivalence point accurately is essential for obtaining reliable results from a titration. While visual methods using indicators provide a quick estimate, calculated methods using derivatives, Gran plots, or software analysis offer higher accuracy and precision. Selecting the most appropriate method requires considering the type of titration, desired accuracy, data quality, and available resources. Understanding the strengths and limitations of each method is crucial for obtaining meaningful results from titration experiments. By carefully analyzing the titration curve and employing the most suitable method, chemists can reliably determine the concentration of unknown solutions. The understanding of equivalence point determination allows for precise control of chemical processes in various fields, from analytical chemistry to industrial applications.
Latest Posts
Latest Posts
-
Lewis Dot Diagram For Ionic Bonds
Mar 20, 2025
-
How Does An Allosteric Inhibitor Work
Mar 20, 2025
-
Rational And Irrational Numbers Practice Problems
Mar 20, 2025
-
A Measure Of The Amount Of Matter In An Object
Mar 20, 2025
-
Enthalpy Heat Of Neutralization For An Acid Base Reaction
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How To Determine The Equivalence Point On A Titration Curve . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
