Enthalpy Heat Of Neutralization For An Acid Base Reaction
Muz Play
Mar 20, 2025 · 6 min read
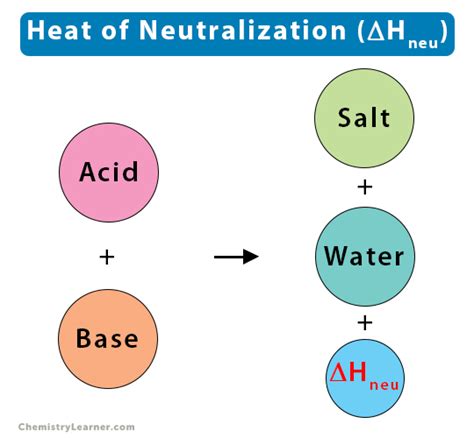
Table of Contents
Enthalpy of Neutralization: A Deep Dive into Acid-Base Reactions
The enthalpy of neutralization, a crucial concept in chemistry, refers to the heat change that occurs during an acid-base reaction. Understanding this heat transfer is vital for comprehending the energetics of chemical reactions and has numerous applications in various fields, from industrial processes to environmental studies. This comprehensive guide will delve into the intricacies of enthalpy of neutralization, exploring its definition, measurement, factors affecting its value, and its significant applications.
What is Enthalpy of Neutralization?
At its core, enthalpy of neutralization is the change in enthalpy (ΔH) that accompanies the reaction between an acid and a base when one mole of water is formed. This is often expressed in kilojoules per mole (kJ/mol). The reaction is typically exothermic, meaning it releases heat to the surroundings, resulting in a negative ΔH value. However, in certain cases, it can be endothermic, absorbing heat from the surroundings and yielding a positive ΔH value.
The fundamental principle behind neutralization lies in the formation of water from hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This equation represents the net ionic equation for a strong acid-strong base neutralization reaction. However, the enthalpy change measured might include contributions from other processes such as ion hydration and changes in the solution's ionic strength.
Strong Acid-Strong Base Neutralization
When a strong acid (e.g., hydrochloric acid, HCl; sulfuric acid, H₂SO₄; nitric acid, HNO₃) reacts with a strong base (e.g., sodium hydroxide, NaOH; potassium hydroxide, KOH), the enthalpy of neutralization is relatively constant, typically around -57 kJ/mol at standard conditions (298 K and 1 atm). This consistency stems from the complete dissociation of both the acid and the base into their respective ions in solution. The reaction is essentially dominated by the formation of water from H⁺ and OH⁻ ions.
Weak Acid-Weak Base Neutralization
The situation becomes considerably more complex when dealing with weak acids and weak bases. The enthalpy of neutralization for these reactions is significantly less negative (or even positive in some instances) compared to strong acid-strong base reactions. This is because only a fraction of the weak acid and weak base molecules dissociate into ions. The overall enthalpy change is then influenced not only by the formation of water but also by the enthalpy changes associated with the ionization of the weak acid and weak base. The heat absorbed or released during the ionization processes significantly affects the net enthalpy of neutralization. Therefore, calculating the enthalpy of neutralization for weak acid-weak base reactions requires considering the equilibrium constants (Ka and Kb) for the acid and base, respectively.
Weak Acid-Strong Base Neutralization & Strong Acid-Weak Base Neutralization
These cases represent intermediate scenarios. The enthalpy of neutralization is less than -57 kJ/mol but more negative than those involving weak acid-weak base reactions. The degree of dissociation of the weak acid or base plays a crucial role in determining the observed enthalpy change. The heat released during the neutralization is partially offset by the heat absorbed during the ionization of the weak acid or base.
Measuring Enthalpy of Neutralization
The experimental determination of the enthalpy of neutralization typically involves calorimetry. A common method is using a coffee-cup calorimeter, a simple apparatus consisting of two nested Styrofoam cups with a lid and a thermometer. This provides a reasonably insulated environment to minimize heat exchange with the surroundings.
The procedure generally follows these steps:
-
Accurately measure the initial temperature of a known volume of acid solution.
-
Add a known volume of base solution to the acid solution, ensuring thorough mixing.
-
Monitor the temperature change over time until a maximum temperature is reached.
-
Calculate the heat absorbed or released by the solution using the following formula:
q = mcΔT
Where:
- q = heat absorbed or released (in Joules)
- m = mass of the solution (in grams)
- c = specific heat capacity of the solution (approximately 4.18 J/g°C for dilute aqueous solutions)
- ΔT = change in temperature (°C)
-
Convert the heat (q) to kilojoules and normalize it to the number of moles of water formed to obtain the enthalpy of neutralization (ΔH).
More sophisticated methods, such as using a bomb calorimeter, provide more accurate measurements by minimizing heat losses and accounting for heat capacity of the calorimeter itself. These are essential for precise determination of enthalpy values, particularly for reactions with smaller heat changes.
Factors Affecting Enthalpy of Neutralization
Several factors can influence the measured enthalpy of neutralization:
- Concentration of reactants: Higher concentrations generally lead to a larger temperature change, but the enthalpy change per mole of water formed remains relatively constant for strong acid-strong base reactions.
- Temperature: Enthalpy changes are temperature-dependent. Accurate measurements need to be made at a controlled temperature, usually standard conditions.
- Nature of the acid and base: The strength of the acid and base significantly impacts the enthalpy of neutralization. Weak acids and bases exhibit lower (less negative) enthalpy changes than strong acids and bases.
- Heat capacity of the solution: The specific heat capacity of the solution affects the temperature change observed for a given heat release or absorption. Different solutions will have different heat capacities.
- Degree of dissociation: The extent of dissociation of weak acids and weak bases is crucial in determining the heat involved in their ionization and consequently affects the overall enthalpy of neutralization.
Applications of Enthalpy of Neutralization
The concept of enthalpy of neutralization has wide-ranging applications:
- Determining the strength of acids and bases: The enthalpy change can provide insights into the relative strengths of acids and bases. A larger (more negative) enthalpy change often indicates stronger reactants.
- Industrial processes: Neutralization reactions are frequently used in industrial settings for wastewater treatment, pH control, and other applications. Understanding the enthalpy changes is crucial for designing efficient and energy-effective processes.
- Environmental studies: Neutralization reactions play a key role in environmental processes, such as acid rain neutralization in soils and water bodies. Knowing the enthalpy change aids in understanding and modelling these environmental processes.
- Thermochemistry studies: Measurements of enthalpy of neutralization provide valuable data for studying thermodynamic properties of chemical reactions and solutions. This data contributes to a deeper understanding of chemical interactions and their energetics.
- Chemical analysis: In certain titrations, the heat released during neutralization can be used to determine the concentration of an unknown solution. The amount of heat generated is directly proportional to the amount of reactant present.
Advanced Considerations
Beyond the basics, a deeper understanding requires consideration of several advanced aspects:
- Ionic strength: The presence of other ions in the solution can influence the enthalpy of neutralization through ion-ion interactions.
- Activity coefficients: In concentrated solutions, the effective concentrations (activities) of ions deviate from their formal concentrations. This must be taken into account for accurate thermodynamic calculations.
- Heat capacity changes: The heat capacity of the solution might change during the reaction, affecting the temperature change measurement.
Conclusion
The enthalpy of neutralization is a fundamental concept with far-reaching applications. While the simple strong acid-strong base reaction provides a straightforward example, understanding the nuances involving weak acids and bases requires a deeper exploration of equilibrium and thermodynamics. Accurate measurement through calorimetry, combined with a nuanced comprehension of the influencing factors, allows for both theoretical advancements and practical applications across numerous scientific and industrial fields. This knowledge is crucial for designing efficient processes, modelling environmental interactions, and further developing our understanding of the energetic landscape of chemical reactions. The study of enthalpy of neutralization continues to be an active area of research, uncovering increasingly sophisticated aspects of acid-base chemistry and its implications.
Latest Posts
Latest Posts
-
What Are The Goals Of Science
Mar 21, 2025
-
Using Punnett Squares To Predict The Outcomes Of Crosses
Mar 21, 2025
-
Is Chemical Energy Kinetic Or Potential Energy
Mar 21, 2025
-
Does Number Of Loops Increase Flux
Mar 21, 2025
-
Is Color A Physical Or Chemical Property
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Enthalpy Heat Of Neutralization For An Acid Base Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
