Is Chemical Energy Kinetic Or Potential Energy
Muz Play
Mar 21, 2025 · 5 min read
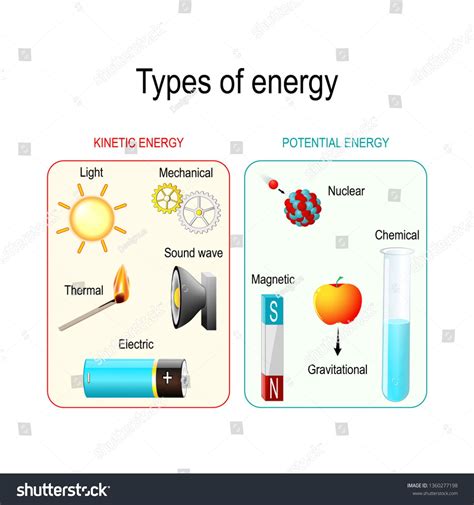
Table of Contents
Is Chemical Energy Kinetic or Potential Energy? Unpacking the Fundamentals
The question of whether chemical energy is kinetic or potential energy is a nuanced one, often sparking debate among students and enthusiasts of chemistry and physics. While a simple answer might seem elusive, a deeper understanding of energy types and their interrelationships reveals a more complete picture. This article delves into the intricacies of chemical energy, exploring its characteristics and relationship to both kinetic and potential energy forms. We'll unravel the complexities, providing clear explanations supported by relevant examples.
Understanding the Different Forms of Energy
Before tackling the central question, let's establish a firm grasp of kinetic and potential energy.
Kinetic Energy: Energy of Motion
Kinetic energy is the energy an object possesses due to its motion. The faster an object moves, the greater its kinetic energy. This is a straightforward concept applicable to macroscopic objects like a rolling ball or a moving car. However, kinetic energy also applies at the microscopic level – the movement of atoms and molecules contributes to the overall kinetic energy of a substance. The formula for kinetic energy is:
KE = 1/2 * mv²
where:
- KE = Kinetic Energy
- m = mass
- v = velocity
Potential Energy: Stored Energy
Potential energy, on the other hand, is stored energy that has the potential to be converted into other forms of energy, such as kinetic energy. Several types of potential energy exist, including:
- Gravitational Potential Energy: Energy stored due to an object's position in a gravitational field. A book held above the ground possesses gravitational potential energy.
- Elastic Potential Energy: Energy stored in a stretched or compressed object, like a spring or a rubber band.
- Chemical Potential Energy: This is the focus of our discussion – energy stored in the chemical bonds of molecules.
Delving into Chemical Potential Energy
Chemical potential energy is the energy stored within the chemical bonds that hold atoms together in molecules. These bonds represent a state of relatively low energy compared to the free, unbound atoms. The formation of chemical bonds releases energy (exothermic reaction), while breaking them requires energy input (endothermic reaction). This stored energy is the crux of chemical reactions.
Think of it this way: Atoms are like magnets with different strengths and polarities. When they bond, they find a stable, lower-energy arrangement, much like magnets snapping together. This energy difference between the unbound and bound state is the chemical potential energy.
Chemical Reactions and Energy Transformations
Chemical reactions involve the breaking and forming of chemical bonds. These transformations lead to a change in the overall potential energy of the system.
-
Exothermic Reactions: In exothermic reactions, the products have lower potential energy than the reactants. The energy difference is released, often as heat or light. Burning fuel is a classic example; the chemical bonds in the fuel are broken, and new bonds are formed in the products (carbon dioxide and water), releasing a significant amount of energy as heat.
-
Endothermic Reactions: Endothermic reactions require energy input to proceed. The products have higher potential energy than the reactants. Photosynthesis is an excellent example; plants absorb sunlight (energy) to convert carbon dioxide and water into glucose (a higher energy molecule).
Therefore, chemical energy is primarily considered potential energy. The energy is stored within the arrangement of atoms and the strength of the bonds holding them together.
The Kinetic Component: Molecular Motion
While chemical energy is fundamentally potential, it's crucial to acknowledge the role of kinetic energy at the molecular level. The atoms and molecules within a substance are constantly in motion, even in a seemingly stationary object. This motion, however, is usually random and disorganized, contributing to the system's thermal energy (heat).
The kinetic energy of molecules influences reaction rates. Higher kinetic energy means molecules move faster, increasing the likelihood of collisions and successful bond-breaking/forming events. However, this kinetic energy is distinct from the potential energy stored in the chemical bonds themselves. It's a contributing factor to the overall energy of the system, but not the primary form of energy involved in chemical reactions.
Examples illustrating the distinction:
Let's consider some everyday examples to solidify our understanding.
1. A Battery: A battery stores chemical potential energy. The chemical reactions within the battery release electrons, creating an electric current—a conversion of chemical potential energy into electrical energy and then kinetic energy (the movement of electrons).
2. Gasoline: Gasoline contains a high amount of chemical potential energy. When combusted in a car engine, this energy is released through chemical reactions, converting it to kinetic energy of the moving pistons and ultimately the car itself.
3. Food: The food we eat is full of chemical potential energy stored in carbohydrates, fats, and proteins. Our bodies break down these molecules, releasing the stored energy and using it to perform various functions – the energy is initially chemical potential, then converted into various other energy forms.
4. Explosives: Explosives are substances that store a tremendous amount of chemical potential energy in their molecular structure. When detonated, the rapid, exothermic chemical reactions transform this potential energy into a powerful burst of kinetic energy – the explosive force.
The Subtle Interplay: Potential and Kinetic in Chemical Processes
The conversion between potential and kinetic energy is a fundamental principle in chemical processes. The initial energy is typically stored as chemical potential energy in the bonds of reactants. As the reaction proceeds, bonds break (requiring energy input or facilitating energy release), and new bonds form, resulting in a shift in the system's overall potential energy. Throughout this process, the kinetic energy of the molecules plays a crucial role in the rate of the reaction, but it remains distinct from the primary source of energy involved – the chemical potential energy.
Conclusion: Chemical Energy is Primarily Potential
In summary, while the kinetic energy of molecules influences reaction rates and the overall energy content of a system, chemical energy is fundamentally potential energy. It's the energy stored in the chemical bonds of molecules, ready to be released or utilized during chemical reactions. Understanding this distinction is crucial for comprehending the principles governing chemical transformations and energy conversions in various physical and biological processes. The interplay between potential and kinetic energy provides a complete picture of energy transformations in chemical systems, revealing a dynamic and fascinating interplay of forces at the molecular level. Future advancements in our understanding of chemical processes will further refine our comprehension of this intricate energy landscape.
Latest Posts
Latest Posts
-
The Basic Functional Unit Of The Kidney Is
Mar 28, 2025
-
Weak Development Of Support Examples In Essay
Mar 28, 2025
-
On The Weak Acid Strong Base Titration Curve
Mar 28, 2025
-
Label The Cranial Dura Septa In The Figure
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Chemical Energy Kinetic Or Potential Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
